Read and report vaccine reactions, harassment and failures.
Measles Disease & Vaccine Information
Measles: The Disease
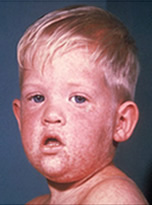
Measles (rubeola) is a highly contagious respiratory disease spread by coughing, sneezing, or simply being in close contact with an infected individual. The disease can be spread even when the rash is not visible. Measles tends to be more severe in children under five and adults over 20. Initial measles symptoms include fever, cough, runny nose, red irritated eyes, and sore throat with tiny white spots on the cheeks inside the mouth (Koplik spots). These symptoms generally last 2-4 days and are followed by the signature itchy red rash on the body around the fourth or fifth day.
Most measles cases in the U.S. resolve without complication, though serious complications can occur. According to the U.S. Centers for Disease Control (CDC), Americans born before 1957 have naturally-acquired immunity to measles through past exposure to the illness. Infants born to mothers with naturally-acquired antibodies to measles benefit from passive maternal immunity. There is also evidence that mothers who have recovered from measles pass short-term measles immunity to their infants by breastfeeding. Learn more about Measles…
Measles Vaccine
The measles vaccine is a weakened (attenuated) form of the live measles virus. There are three live virus vaccines currently available for use in the U.S.: Merck's MMRII vaccine containing measles, mumps, and rubella attenuated live viruses; Merck's Proquad (MMRV) vaccine containing measles, mumps, rubella, and varicella attenuated live viruses; and GlaxoSmithKline’s PRIORIX containing measles, mumps, and rubella attenuated live viruses. The CDC currently recommends that children receive two doses of a measles containing vaccine, with the first dose administered between 12-15 months and the second dose between 4-6 years. The CDC also recommends that individuals born after 1957 who have no laboratory evidence of immunity or documentation of vaccination should receive at least one dose of MMR vaccine. Learn more about Measles vaccine…
Measles Quick Facts
Measles
- After coming in contact with someone infected with measles, the incubation period to onset of the rash is between 7 and 21 days, with an average of 14 days. The period leading up to the appearance of the rash is characterized by a rising fever that peaks at 103-105 degrees F.
- Three years beforethe first measles vaccine became available in 1960, there were approximately 442,000 reported measles cases and 380 related deaths in the U.S. among the 3.5 to 5 million Americans likely infected with measles. Measles-associated deaths are rare in the U.S., with the last reported death occurring in 2015. Continue reading quick facts…
Measles Vaccine
- There are three measles vaccines currently in use in the United States. Two vaccines, MMRII and PRIORIX, are combination measles-mumps-rubella (MMR) live virus vaccines. The third, ProQuad, is a combination measles-mumps-rubella-varicella (MMR-V) live virus vaccine. Both MMRII and ProQuad are manufactured and distributed by Merck, while PRIORIX is manufactured and distributed by GlaxoSmithKline (GSK). The CDC recommends children receive the first dose of MMR vaccine between 12 and 15 months and the second dose between 4 and 6 years.
- As of March 29, 2024, there have been 112,733 reports of measles-vaccine reactions, hospitalizations, injuries, and deaths following measles vaccinations made to the federal Vaccine Adverse Events Reporting System (VAERS), including 560 related deaths, 8,639 hospitalizations, and 2,162 related disabilities. Over 50 percent of those adverse events occurred in children three years old and under. Continue reading quick facts...
NVIC encourages you to become fully informed about Measles and the Measles vaccine by reading all sections in the Table of Contents below, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.