Read and report vaccine reactions, harassment and failures.
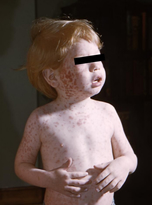
The U.S. Centers for Disease Control (CDC) report minor side effects from the MMR-V and MMR vaccines to include low-grade fever, injection site redness or rash, pain at the injection site, and facial swelling. Moderate side effects include a full body rash, temporary low platelet count, temporary stiffness and pain at the joints, and seizures. A vaccine strain infection following vaccination may be the cause of the full body rash.
There is a significantly greater risk of seizures following MMR-V vaccine in comparison to separate administrations of MMR and varicella vaccines if the MMR-V is given as the first dose of the series.
Rare serious side effects of both MMR-V and MMR include seizures, life-threatening infection, severe allergic reaction, and death.
Serious complications reported by Merck in the MMR-V (ProQuad) product insert during vaccine post-marketing surveillance have included:
- measles;
- atypical measles;
- vaccine strain varicella;
- varicella-like rash;
- herpes zoster;
- herpes simplex;
- pneumonia and respiratory infection;
- pneumonitis;
- bronchitis;
- epididymitis;
- cellulitis;
- skin infection;
- subacute sclerosing panencephalitis;
- aseptic meningitis;
- thrombocytopenia;
- aplastic anemia (anemia due to the bone marrow’s inability to produce platelets, red and white blood cells);
- lymphadenitis (inflammation of the lymph nodes);
- anaphylaxis including related symptoms of peripheral, angioneurotic and facial edema;
- agitation;
- ocular palsies;
- necrotizing retinitis (inflammation of the eye);
- nerve deafness;
- optic and retrobulbar neuritis (inflammation of the optic nerve);
- Bell’s palsy (sudden but temporary weakness of one half of the face);
- cerebrovascular accident (stroke);
- acute disseminated encephalomyelitis;
- measles inclusion body encephalitis;
- transverse myelitis;
- encephalopathy;
- Guillain-Barre Syndrome;
- syncope (fainting);
- tremor;
- dizziness;
- paraesthesia;
- febrile seizure;
- afebrile seizures or convulsions;
- polyneuropathy (dysfunction of numerous peripheral nerves of the body);
- Stevens-Johnson syndrome;
- Henoch-Schönlein purpura;
- acute hemorrhagic edema of infancy;
- erythema multiforme;
- panniculitis;
- arthritis;
- death
A 2014 published study on the MMR-V vaccine in Canada found that the risk of febrile seizures to be double in children receiving the MMR-V vaccine in comparison to those receiving separate doses of MMR and varicella vaccines. A 2015 meta-analysis found a two-fold increase in febrile seizures between 5 and 12 days or 7 and 10 days following MMR-V vaccination in children between the ages of 10 and 24 months.
MMR-V vaccine contains albumin, a human blood derivative, and as a result, a theoretical risk of contamination with Creutzfeldt-Jakob Disease (CJD) exists. Merck states that no cases of transmission of CJD or other viral diseases have been identified and virus pools, cells, bovine serum, and human albumin used in vaccine manufacturing are all tested to assure the final product is free of potentially harmful agents.
Serious complications reported by Merck in the MMRIIproduct insert during vaccine post-marketing surveillance have included:
- brain inflammation (encephalitis) and encephalopathy (chronic brain dysfunction);
- panniculitis (inflammation of the fat layer under the skin);
- atypical measles;
- syncope (sudden loss of consciousness, fainting);
- vasculitis (inflammation of the blood vessels);
- pancreatitis (inflammation of the pancreas);
- diabetes mellitus;
- thrombocytopenia purpura (blood disorder);
- Henoch-Schönlein purpura (inflammation and bleeding in the small blood vessels);
- acute hemorrhagic edema of infancy (rare vasculitis of the skin’s small vessels occurring in infants);
- leukocytosis (high white blood cell count);
- anaphylaxis (shock);
- bronchial spasms;
- pneumonia;
- pneumonitis(inflammation of the lung tissues);
- arthritis and arthralgia (joint pain);
- myalgia (muscle pain);
- polyneuritis (inflammation of several nerves simultaneously);
- measles inclusion body encephalitis (disease affecting the brain of immunocompromised persons);
- subacute sclerosing panencephalitis (fatal progressive brain disorder thought to be caused by exposure to the measles virus);
- Guillain-Barre Syndrome (disease where the body’s immune system attacks the nerves);
- acute disseminated encephalomyelitis (ADEM- brief widespread inflammation of the nerve’s protective covering);
- transverse myelitis (inflammation of the spinal cord);
- aseptic meningitis;
- erythema multiforme (skin disorder from an allergic reaction or infection);
- urticarial rash (hives, itching from an allergic reaction);
- measles-like rash;
- Stevens-Johnson syndrome (severe reaction causing the skin and mucous membranes to blister, die, and shed);
- nerve deafness (hearing loss from damage to the inner ear);
- otitis media (ear infection);
- retinitis (inflammation of the retina of the eye);
- optic neuritis (inflammation of the optic nerve);
- conjunctivitis (pink eye);
- ocular palsies (dysfunction of the ocular nerve);
- epididymitis (inflammation of the epididymis);
- paresthesia (burning or prickling of the skin);
- death.
Serious complications reported by GlaxoSmithKline in the PRIORIX package insert during vaccine post-marketing surveillance have included:
- Vasculitis (including Henoch-Schönlein purpura and Kawasaki syndrome);
- Thrombocytopenia and thrombocytopenic purpura;
- Anaphylactic reactions;
- Meningitis;
- “Mumps like” illness;
- “Measles like” illness;
- Orchitis;
- Epididymitis;
- Parotitis;
- Erythema multiforme;
- Arthralgia;
- Arthritis;
- Encephalitis;
- Cerebellitis;
- Cerebellitis-like symptoms (including transient gait disturbance and transient ataxia);
- Guillain-Barré syndrome;
- Transverse myelitis;
- Peripheral neuritis;
- Afebrile seizures;
In the comprehensive report evaluating scientific evidence, Adverse Effects of Vaccines: Evidence and Causality, published in 2012 by the Institute of Medicine (IOM), 30 reported vaccine adverse events following the Measles, Mumps, and Rubella (MMR) vaccine were evaluated by a physician committee. These adverse events included measles inclusion body encephalitis, febrile seizures, arthritis, meningitis, Guillain-Barre Syndrome, autism, diabetes mellitus, optic neuritis, transverse myelitis and more.
In 23 of the 30 measles, mumps, and rubella (MMR) vaccine-related adverse events evaluated, the IOM committee concluded that there was inadequate evidence to support or reject a causal relationship between the MMR vaccine and the reported adverse event, primarily because there was either an absence of methodologically sound published studies or too few quality studies to make a determination.
The IOM committee, however, concluded that the scientific evidence “convincingly supports” a causal relationship between febrile seizures, anaphylaxis, and measles inclusion body encephalitis in immunocompromised individuals and the MMR vaccine and favored acceptance of a causal relationship between transient arthralgia in both children and women and the MMR vaccine. After reviewing only five epidemiological studies, the IOM committee concluded that it favored rejection of a causal association between both autism and Type 1 diabetes and the MMR vaccine.
In 2012, the Cochrane Collaborative examined 57 studies and clinical trials involving approximately 14.7 million children who had received the MMR vaccine. While the study authors stated that they were not able to detect a “significant” association between MMR vaccine and autism, asthma, leukemia, hay fever, type I diabetes, gait disturbance, Crohn’s disease, demyelinating diseases or bacterial or viral infections, they reported that “The design and reporting of safety outcomes in MMR vaccine studies, both pre- and post-marketing, are largely inadequate.”
In an updated review published in April of 2020, the Cochrane Collaborative reviewed 87 safety studies associated with MMR, MMR-V, and MMR + Varicella vaccine. This review concluded that there was an association between MMR vaccines containing Leningrad-Zagreb and Urabe mumps strains and aseptic meningitis, but no evidence to support this association for MMR vaccines which contain the Jeryl Lynn mumps strains. This conclusion was based on the evaluation of nine studies, all of which were considered low certainty studies. The Cochrane Collaborative reports low certainty studies to be those where their “confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.”
An association was also found between MMR vaccines and idiopathic thrombocytopenic purpura (ITP) and MMR, MMR-V, and MMR + Varicella vaccines and febrile seizures and the studies evaluated to make this determination were a combination of both moderate certainty and low certainty studies. Moderate certainty studies are those in which the Cochrane Collaborative are “moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.”
The Cochrane Collaborative reported that no association was found between MMR vaccine and encephalitis, encephalopathy, cognitive delay, type 1 diabetes, asthma, dermatitis/eczema, hay fever, leukemia, multiple sclerosis, gait disturbance, and bacterial or viral infections; however, all the studies that were evaluated were found to be either low certainty or very low certainty studies. Very low certainty studies are those in which the Collaborative have “very little confidence in the effect estimate” and that “the true effect is likely to be substantially different from the estimate of effect.”
No association was found between autism spectrum disorders and MMR vaccines, and the studies evaluated were a combination of moderate certainty and low certainty studies. The Cochrane Collaborative also reported that there was insufficient evidence to support or reject an association between MMR vaccines and inflammatory bowel disease.
As of March 29, 2024, there have been 112,733 reports of measles-vaccine reactions, hospitalizations, injuries, and deaths following measles vaccinations made to the federal Vaccine Adverse Events Reporting System (VAERS), including 560 related deaths, 8,639 hospitalizations, and 2,162 related disabilities. However, the numbers of vaccine-related injuries and deaths reported to VAERS may not reflect the true number of serious health problems that occur after measles vaccination.
Even though the National Childhood Vaccine Injury Act of 1986 legally required pediatricians and other vaccine providers to report serious health problems following vaccination to federal health agencies (VAERS), many doctors and other medical workers giving vaccines to children and adults fail to report vaccine-related health problem to VAERS. There is evidence that only between 1 and 10 percent of serious health problems that occur after use of prescription drugs or vaccines in the U.S. are ever reported to federal health officials who are responsible for regulating the safety of drugs and vaccines and issue national vaccine policy recommendations.
As of April 1, 2024, there have been 1,367 claims filed in the federal Vaccine Injury Compensation Program (VICP) for 85 deaths and 1,282 injuries that occurred after measles vaccination. Of that number, the U.S. Court of Claims administering the VICP has compensated 526 children and adults, who have filed claims for measles vaccine injury.
One example of an MMR vaccine injury claim awarded compensation in the VICP is the case of O.R. On February 13, 2013, O.R. received the MMR, Haemophilus Influenzae type B (Hib), Pneumococcal (Prevnar 13), Hepatitis A, and Varicella vaccines. That evening following vaccination, she became feverish and irritable, which prompted her mother to contact the doctor. The doctor advised O.R.’s mom to administer Benadryl and Tylenol for her symptoms. The fever persisted for several days and was followed by a severe seizure resulting in cardiac and respiratory arrest. The cardiac arrest and seizure caused O.R. to develop encephalopathy, kidney failure, severe brain injury, low muscle tone and cortical vision impairment. After several months of inpatient hospitalization, O.R. was discharged home with 24-hour supervised medical care. On November 20, 2017, the court conceded that the MMR vaccine caused her encephalopathy and O.R. was awarded a $101 million-dollar settlement to cover medical expenses for the rest of her life.
In 1998, public health officials and attorneys associated with the federal Vaccine Injury Compensation Program published a review in Pediatrics in regard to the medical records of 48 children ages 10 to 49 months, who received a measles vaccine or combination MMR vaccine between 1970 and 1993 and developed encephalopathy after vaccination. The children either died or were left with permanent brain dysfunction, including developmental regression and delays, chronic seizures, motor and sensory deficits and movement disorders. The study authors concluded that:
“The onset of neurologic signs or symptoms occurred with a nonrandom, statistically significant distribution of cases on days 8 and 9. No cases were identified after the administration of monovalent mumps or rubella vaccine. This clustering suggests that a causal relationship between measles vaccine and encephalopathy may exist as a rare complication of measles vaccination.”
Nearly two decades earlier, in 1981, a report of the National Childhood Encephalopathy Study was published in Britain that concluded:
“The risk of a serious neurological disorder within 14 days after measles vaccine in previously normal children irrespective of eventual clinical outcome is 1 in 87,000 immunizations.”
However, a 2007 study conducted in Britain concluded We can estimate the vaccine-attributable risk of serious neurologic disease after the first dose of MMR vaccine as 1 in 365,000 doses
.
Published studies have also found that the MMR vaccine components or excipients, particularly egg antigens and porcine or bovine gelatin, can trigger both immediate and delayed anaphylactic reactions.
In Guinea-Bissau, Dr. Peter Aaby has studied and administered vaccines to thousands of children for more than three decades and has published research on vaccine safety and effectiveness, including research on measles and measles vaccine. In 2003, Dr. Aaby published a paper noting differences in the way that boys and girls respond to vaccines and reported that there was an increased risk of mortality in girls who received DTP and measles vaccines at the same time. Further, in 2007, he also found that fatality rates were increased for children 6 to 17 months of age, if they had received the DTP vaccine with or after the measles vaccine.
In 1995, Swiss researchers discovered the presence of the reverse transcriptase (RT) enzyme in the live measles and mumps vaccine, and traced it back to the cells of the chickens used to create the vaccine. Reverse transcriptase is responsible for copying RNA into DNA and its activity is associated with the presence of retroviruses, a class of viruses which has the ability to permanently alter the genes of the cells they infect.
While the World Health Organization (WHO) and the CDC reviewed the findings, they were also quick to dismiss them, with the CDC publicly stating that we are not investigating a situation in which there has been any adverse reaction at all.
Independent researchers have expressed concerns that the use of animal tissues for the production of human vaccines such as the live MMR vaccine may facilitate the transfer of viral infection from animals into man causing as yet undetected and unevaluated negative health effects on humans.
The first evidence of persistent measles virus infection of the intestine after measles vaccination was discovered in 1995 by British researchers. In 1998, an association between live virus measles vaccine, inflammatory bowel disease (IBD) and regressive autism was hypothesized by gastroenterologist Dr. Andrew Wakefield and his colleagues at UK’s Royal Free Hospital following the detection of measles in the intestines of children suffering with Crohn’s disease and autism. His paper, published in The Lancet, which suggested MMR vaccine may be associated with the development of regressive autism in previously healthy children, was met with intense anger and criticism from public health officials and medical trade associations.
Hans Asperger had observed a high rate of gastrointestinal (celiac) disease in children diagnosed with autism, and his observation prompted further investigation by Wakefield and his colleagues. After studying children at the Royal Free Hospital who were suffering from inflammatory bowel disease, the researchers hypothesized that a persistent viral infection, either from natural measles disease or live virus measles vaccine, could cause chronic inflammation in the bowel and even damage to the central nervous system in susceptible children.
In their published paper, Wakefield and his colleagues emphasized that they had not proven a cause and effect relationship between autism, MMR vaccine and non-specific colitis, which they referred to as autistic ileal-lymphoid-nodular hyperplasia, but rather they called for more studies to explore the potential relationship.
Additional independent studies on this subject have also reported the presence of measles virus in association with gastrointestinal disorders, such as enterocolitis and chronic intestinal inflammation.
Today, most doctors and health officials reject the suggestion that MMR vaccine is associated with the development of autism in children. However, privately funded research continues to investigate the potential association between vaccines, including MMR vaccine, and the development of autism, inflammatory bowel disease and additional brain and immune system dysfunction in previously healthy children.
In April 2019, the Informed Consent Action Network (ICAN), a non-profit which investigates the safety of medical procedures, pharmaceutical drugs and vaccines while educating the public on their right to informed consent of all medical procedures, received documentation from the Food and Drug Administration (FDA) in response to their Freedom of Information Act (FOIA) request for the clinical studies pertaining to the MMRII vaccine approval.
The documents provided to ICAN revealed that only eight pre-licensing clinical trials involving a total of 834 children who were followed for only 42 days were completed prior to MMRII vaccine licensing. Of the eight studies, three studies of less than 350 children compared the MMRII vaccine to another vaccine. The remaining five studies compared health outcomes of children vaccinated with different lots of MMRII vaccine.
In all eight pre-clinical licensing studies, high rates of upper respiratory illness (55 percent) and gastrointestinal illness (40 percent) were reported, along with additional side effects which included fever, malaise, and measles-like rash.
The MMRII and the MMR-V product inserts report the following:
- Measles inclusion body encephalitis, pneumonitis, and death have occurred in severely immunocompromised individuals who were inadvertently vaccinated. Disseminated mumps and rubella infections have also been reported in this population.
- Subacute sclerosing panencephalitis (SSPE) has been reported in children without a history of wild-type measles infection, however, these children were documented to have received measles vaccine. The vaccine product insert speculates that some cases may have either resulted from measles vaccination or from a possible unrecognized case of measles in the first year of life.
- In the majority of susceptible individuals, small amounts of the live attenuated rubella virus have been excreted from the throat or nose seven to 28 days following vaccination. According to the vaccine product insert, no evidence has confirmed that the rubella virus can be transmitted to susceptible individuals who come into contact with vaccinated persons. Transmission through close personal contact has been accepted as being theoretically possible but it is not considered a significant risk.
- Transmission of the rubella vaccine virus through breast milk has been noted and postpartum women vaccinated with a live attenuated rubella vaccine may transmit the virus to their breast-fed infants. In one study, several infants were found to have serological evidence of rubella infection without severe disease and one infant was noted to have a mild illness found to be typical of rubella.
- Vaccine product inserts for MMR and MMR-V deny any reports of transmission of live attenuated mumps or measles viruses from persons vaccinated and susceptible close contacts. It is not known whether the measles or mumps vaccine virus secretion in human milk.
In November 2014, the National Vaccine Information Center published a special report The Emerging Risks of Live Virus and Virus Vectored Vaccines: Vaccine Strain Virus Infection, Shedding and Transmission. This report reviewed the medical literature for evidence that live virus vaccine strain infection, shedding, and potential for transmission occurs, including measles vaccine strain infection and shedding.
There have been published reports of vaccine-strain measles infection with clinical symptoms that are indistinguishable from wild-type measles. There are also a few reports of measles vaccine-strain virus shedding and lab confirmed infection in children following MMR vaccination.
In 2002, there was a published report by researchers in France of a child presenting with fever 8 days after vaccination with a measles-mumps-rubella vaccine. Measles virus was isolated in a throat swab taken 4 days after fever onset. This virus was then further genetically characterized as a vaccine-type virus.
In 2010, Eurosurveillance published a report about the shedding of vaccine-strain measles virus in urine and throat secretions of a Croatian child with vaccine-associated rash illness. A healthy 14-month old child was given MMR vaccine and eight days later developed macular rash and fever. Lab testing of throat and urine samples between two and four weeks after vaccination tested positive for vaccine strain measles virus. Authors of the report pointed out that when children experience a fever and rash after MMR vaccination, only molecular lab testing can determine whether the symptoms are due to vaccine strain measles virus infection.
They stated:
“According to WHO guidelines for measles and rubella elimination, routine discrimination between aetiologies of febrile rash disease is done by virus detection. However, in a patient recently MMR-vaccinated, only molecular techniques can differentiate between wild type measles or rubella infection or vaccine-associated disease. This case report demonstrates that excretion of Schwartz measles virus occurs in vaccinees.”
In 2012, a report was also published describing a healthy 15-month old child in Canada, who developed irritability, fever, cough, conjunctivitis and rash within seven days of an MMR shot. Blood, urine and throat swab tests confirmed a vaccine strain measles virus infection 12 days after vaccination. Addressing the potential for measles vaccine strain virus transmission to others, the authors stated:
“While the attenuated virus can be detected in clinical specimens following immunization, it is understood that administration of the MMR vaccine to immunocompetent individuals does not carry the risk of secondary transmission to susceptible hosts.”
IMPORTANT NOTE: Even though ACIP says it’s safe to give other viral and bacterial vaccines at the same time as MMR vaccine, Merck’s MMRII product information insert states that other live virus vaccines—such as varicella should NOT be given at the same time as MMR vaccine but rather should be administered one month prior or one month after MMR vaccination.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Measles and the Measles vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.



