Read and report vaccine reactions, harassment and failures.
Pneumococcal (S. Pneumoniae)
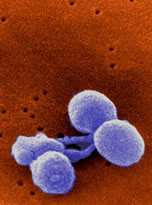
- Pneumococcal disease is a bacterial infection caused by S. pneumoniae (Streptococcus pneumoniae). It is the most common cause of bacterial pneumonia and middle ear infection (otitis media) in the U.S., and is the third most frequent cause of bacterial meningitis. Pneumococcal bacteria are often found in the upper area of the throat behind the nose in about 5 to 90 percent of healthy individuals. However, when pneumococcal bacteria cause invasive infection, serious complications can lead to inflammation of the brain, blood infections, pneumonia and death.
- Symptoms of pneumococcal infection include sudden onset of fever and fatigue, sneezing and cough with mucus and shortness of breath. The infection may start with a general feeling of being unwell, a low-grade fever and a cough that doesn’t include mucus before symptoms worsen. Symptoms of pneumococcal meningitis (brain inflammation) include stiff neck (inability to touch the chin to chest without moderate to severe pain in the back of the neck and head); headache; extreme fatigue or seizures. Symptoms of otitis media include a painful ear, red or swollen eardrum, fever, and irritability.
- Pneumococcal bacteria are primarily transmitted through respiratory secretions by coughing and sneezing. Persons most at risk of developing invasive pneumococcal disease include immunocompromised individuals, smokers, persons with chronic cardiac, lung, or kidney disease, individuals without a spleen, and persons with cochlear implants or a cerebrospinal fluid leak. Children attending daycare are also at a higher risk.
- Otitis media, often referred to as a middle ear infection, is commonly caused by S. pneumoniae and remains the most common pediatric infection requiring treatment by the age of 12 months. Over 60 percent of children will experience at least one episode of acute otitis media prior to the age of one. S. pneumoniae is the leading cause of bacterial meningitis in children under the age of five. Pneumococcal pneumonia, the most common infection caused by S. pneumoniae in adults, is estimated to cause over 400,000 hospitalizations each year in the United States. 36 percent of all community acquired pneumonias are caused by S. pneumoniae.
- Globally, 16 percent of deaths worldwide in children under 5 are related to pneumonia, with most deaths occurring in sub-Saharan Africa and south Asia. In the United States, the CDC combines the death rates of pneumonia with influenza and estimates it to be the eighth leading cause of deaths in persons 65 years of age or older. In 2017, there were 19,620 reported cases of invasive S. pneumoniae in the U.S., with 1,220 cases occurring in children under the age of five.
Pneumococcal Vaccine
- There are two types of pneumococcal vaccines licensed for use in the U.S. today: Pneumovax 23, a pneumococcal polysaccharide vaccine (PPSV23) manufactured by Merck, and Prevnar 13, a pneumococcal conjugate vaccine (PCV13) manufactured by Wyeth (Pfizer) pharmaceuticals. PPSV23 contains 23 strains of pneumococcal and is approved for use in adults 50 and older and in children 2 and older who are at an increased risk for pneumococcal disease. PCV13) vaccine contains 13 strains of pneumococcal and is approved for the prevention of otitis media and invasive disease caused by S. pneumoniae in children between 6 weeks and 5 years of age. It is also approved for the prevention of invasive disease caused by S. pneumoniae in children between 6 and 17 years and for the prevention of pneumonia and invasive disease caused by S. pneumoniae in adults 18 years of age and older.
- The CDC recommends four doses of pneumococcal conjugate vaccine (PCV13) for infants and children, with a dose given at 2, 4, 6 and between 12 and 18 months of age. Children between 2 and 18 years who are at a higher risk of invasive pneumococcal disease are also recommended to receive one dose of PPSV23 at least 8 weeks following the most recent dose of PCV13. A second dose of PPSV23 is recommended at least 5 years following the first dose in children who are immunocompromised, HIV-positive, have sickle cell disease, or who lack a functioning spleen. PPSV23 and PCV13 are also recommended for use in immunocompromised children, adults, and seniors.
- All adults 65 years of age and older are recommended by the CDC to receive one dose of PPSV23. PCV13 can also be considered for use in healthy seniors 65 years and older but routine vaccination is no longer recommended. This recommendation was revised at the June 2019 Advisory Committee on Immunization Practices (ACIP) when data presented found limited benefit to vaccinating all seniors with PCV13 vaccine.
- According to the CDC, PCV7, the original pneumococcal conjugate vaccine, resulted in a 97 percent decrease in invasive pneumococcal disease caused by the 7 pneumococcal strains found within the vaccine. However, the mass use of PCV7 vaccine by American children put pressure on some of the nearly 90 additional pneumococcal strains known to cause invasive disease resulting in an increased rate of otitis media from by serotypes not included in the seven-valent vaccine. In an attempt to prevent 6 additional pneumococcal strains from causing invasive disease, PCV13 vaccine was developed to replace PCV7. Current research indicates that while PCV13 has significantly decreased nasopharyngeal colonization with the serotypes found within the vaccine, replacement with non-vaccine type strains continues. New vaccines to target emerging strains of pneumococcal not covered in the current vaccines are currently in clinical trials.
- Reported pneumococcal vaccine reactions include fever, severe local reactions (swelling, redness, and pain at site of injection), irritability, drowsiness, restless sleep, vomiting, diarrhea, rash, decreased appetite, convulsions, asthma, pneumonia and sudden infant death syndrome (SIDS).
- Using the MedAlerts search engine, as of March 29, 2024, there have been 27,377 serious adverse events reported to the Vaccine Adverse Events Reporting System (VAERS) in connection with pneumococcal vaccinations (PCV7, PCV13, PCV15, PCV20, and PPSV23). Over 57 percent of these reported serious pneumococcal vaccine-related adverse events occurred in children 6 and under. Of these pneumococcal-vaccine related adverse event reports to VAERS, 2,728 were deaths, with nearly 66 percent occurring in children under 6 years of age.
- As of April 1, 2024, there have been 385 claims filed in the federal Vaccine Injury Compensation Program (VICP) for injuries and deaths following vaccination with pneumococcal conjugate vaccine (PCV), including 26 deaths and 359 serious injuries. Pneumococcal polysaccharide vaccine (PPSV23) is not covered under the federal Vaccine Injury Compensation Program (VICP) and compensation for injuries and deaths related to vaccination with PPSV23 are pursued in civil court.
Food & Drug Administration (FDA)
- Pneumococcal Vaccine, Polyvalent Merck & Co. Inc. Pneumovax 23 Licensing Information
- Pneumococcal 7-Valent Conjugate Vaccine (Diphtheria CRM197Protein) Pfizer/ Pharmaceuticals Inc. Prevnar Licensing Information
- Pneumococcal 13-Valent Conjugate Vaccine (Diphtheria CRM197Protein) Pfizer/Wyeth Pharmaceuticals Inc. Prevnar 13 Licensing Information
Centers for Disease Control (CDC)
- CDC on Pneumococcal Disease
- CDC on Pneumococcal Vaccination
- CDC Vaccine Information Sheets: Pneumococcal 13-Valent Conjugate Vaccine (Prevnar) and Pneumococcal Polysaccharide Vaccine (Pneumovax 23)
Vaccine Reaction Symptoms & Ingredients
Our Ask 8, If You Vaccinate webpage contains vaccine reaction symptoms and more.
Search for Vaccine Reactions
NVIC hosts MedAlerts, a powerful VAERS database search engine. MedAlerts examines symptoms, reactions, vaccines, dates, places, and more.
Reporting a Vaccine Reaction
Since 1982 the NVIC has operated a Vaccine Reaction Registry, which has served as a watchdog on VAERS. Reporting vaccine reactions to VAERS is the law. If your doctor will not report a reaction, you have the right to report a suspected vaccine reaction to VAERS.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Pneumococcal and the Pneumococcal vaccine by reading all sections in the Table of Contents , which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.



