Read and report vaccine reactions, harassment and failures.
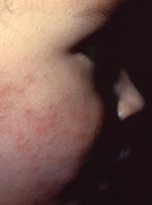
According to the CDC, mild side effects such as redness, warmth, or swelling where the shot was given have been reported in connection with administration of Hib vaccines. Fever has also been reported following vaccination. Other adverse events that may occur include dizziness, fainting, allergic reaction, other serious injury and death.
In pre-licensing clinical trials, the severity of adverse reactions varied depended on which vaccine was given, as well as the additional vaccines administered simultaneously. Some of the events reported by the manufacturers included:
ActHIB — tenderness, erythema, swelling, induration, fever, irritability, drowsiness, anorexia, unusual or persistent crying, vomiting, and seizure with apnea. Reported side effects post marketing have included convulsions, anaphylaxis, peripheral edema, extensive limb swelling, pruritus, and rash. Initial pre-licensing clinical safety studies of ActHIB compared the vaccine against other vaccines such as hepatitis B, while other studies involved ActHIB to be administered in addition to multiple vaccines including the DTP vaccine. Additional clinical studies on ActHIB involved the solicitation of adverse events when the vaccine was administered along with DTaP, IPV (polio), PCV7, and hepatitis B vaccines. No clinical studies involved a comparison of ActHIB against a true placebo.
HIBERIX —redness, pain and swelling at injection site, fever, fussiness, irritability, loss of appetite, restlessness, sleepiness, diarrhea, vomiting, seizure, bilateral pneumonia; Reported side effects post marketing have included extensive swelling of the vaccinated limb, anaphylactic reactions, angioedema, convulsions, hypotonic-hyporesponsive episode, syncope, apnea, rash, urticaria, somnolence. Pre-licensing clinical trials of HIBERIX did not involve the use of a true placebo and most trials involved the testing of HIBERIX against another licensed Hib vaccine in combination with multiple additional vaccines. For example, one pre-licensing clinical safety study compared HIBERIX to another U.S. licensed Hib vaccine administered along with PEDIARIX vaccine (combination DTaP, inactivated polio, and hepatitis B vaccine). Study participants who received HIBERIX or another U.S. licensed Hib vaccine also received a dose of PEDIARIX and a dose of PCV13 (13-valent pneumococcal vaccine) at all three doses AND a dose of ROTARIX live oral rotavirus vaccine at dose 1 and 2. Study participants receiving PEDIARIX also received a dose of PCV13 and a dose of ENGERIX-B (Hepatitis B vaccine) when indicated, dependent on whether a dose of hepatitis B vaccine was administered at birth.
PedvaxHIB —irritability, sleepiness, injection site pain/soreness, injection site swelling/induration, injection site erythema, prolonged crying (more than 4 hours), unusual high-pitched crying, vomiting, diarrhea, pain, crying, otitis media, rash, upper respiratory infection, seizures, tracheitis, thrombocytopenia, urticaria; potential adverse events may also include early onset of Hib disease and Guillain-Barre syndrome (GBS); Reported side effects post marketing have included lymphadenopathy, febrile seizures, angioedema, and injection site abscess. Pre-licensing clinical trials of liquid PedvaxHIB compared the vaccine to lyophilized PedvaxHIB with most children in each study group receiving the DTP and oral polio vaccine in addition to PedvaxHIB. No pre-licensing clinical trials involved the comparison of PedvaxHIB against a true placebo.
Pentacel — irritability, fussiness, inconsolable crying, injection site redness, swelling, tenderness, increase in arm circumference (dose 4), fever, lethargy, hypotonic hyporesponsive episodes (HHE), seizures, febrile seizures, bronchiolitis, dehydration, pneumonia, gastroenteritis, asthma, pneumonia, encephalopathy, and four deaths attributed to asphyxia due to suffocation, head trauma, sudden infant death syndrome (SIDS), and neuroblastoma. Reported side effects post marketing have included cyanosis, vomiting, diarrhea, extensive swelling of injected limb including swelling that involved adjacent joints, invasive Hib disease (classified as vaccine failure), rash, urticarial, meningitis, rhinitis, viral infection, decreased appetite, somnolence, hypotonic hyporesponsive episodes, depressed level of consciousness, screaming, apnea, cough, erythema, skin discoloration, and pallor. In pre-licensing clinical safety trials of Pentacel, there were no true placebo studies and most clinical trials compared the product against multiple vaccines including DTaP (diphtheria, tetanus, and acellular pertussis vaccine), polio vaccine, and ActHIB (Hib conjugated vaccine). In addition to the administration of Pentacel or the control vaccine(s), study participants also received PCV7 (7-valent pneumococcal vaccine, hepatitis B vaccine, and in some trials, measles, mumps, rubella (MMR) and varicella vaccines were also administered. Additionally, one pre-licensing clinical safety studies did not have a control group and involved the evaluation of adverse events reported following the fourth dose of Pentacel.
Pentacel was first licensed in Canada in May of 1997, and data reported from Canada was evaluated by the FDA as part of the licensing process. In Canada, between May 1, 1997 and April 30, 2006, there were 245 medically confirmed adverse events reported to the manufacturer, with 237 adverse events reports submitted by health care authorities and providers and eight reports pulled from literature. There were also 43 adverse events reported from health care consumers. Adverse events included: vomiting; diarrhea; cyanosis; eye rolling; injection site reactions including pain, inflammation, mass, and abscess; fever, irritability; edema, including peripheral edema; decreased vaccine response and vaccine failure; sudden infant death syndrome; sudden death; viral infection; rhinitis; meningitis; decreased appetite; seizures; hypotonic-hyporesponsive episode; somnolence; hypotonia; lethargy; encephalopathy; depressed level of consciousness; rash, including maculo-papular; skin discoloration; urticaria; and pallor. Fourteen deaths were reported following the administration of Pentacel and causes included Sudden Infant Death Syndrome (5 cases), unknown causes (4 cases), invasive Hib disease (2 cases), sepsis (1 case), convulsions (1 case), congenital anomaly (1 case).
In addition to the deaths that occurred during the Pentacel clinical trials, deaths following the administration of other pentavalent vaccines have also been reported. In a March 2012 open letter to Dr. Margaret Chan, then director general of the World Health Organization, the letter’s authors called attention to the deaths connected with the pentavalent (DPT + Hib + Hepatitis B) vaccine in India, Sri Lanka, Bhutan, and Pakistan and noted the cause of the problem to be unrelated to the brand, manufacturer or lot of the vaccine, while stating that:
“It appears to be a form of ‘hypersensitivity reaction’ as described in the post mortem report on one of the children in Kerala. The vaccine can be administered to many patients without problems and there is no available method at present to predict which infant will react adversely.”
Several studies on a Hib containing pentavalent vaccine used in South Asia and India have reported an elevated risk of death following vaccination, with several physicians publicly rejecting the use of the pentavalent vaccine.
In a pediatric safety and use review of Pentacel presented in March of 2010, Dr. Jane Woo with the FDA discussed reported adverse reactions related to the vaccine following the June 2008 FDA approval. At the meeting, Dr. Woo reported that 775 adverse reactions, 170 of which were considered serious including 26 deaths, had been reported to VAERS in connection with Pentacel between June 20, 2008 (when Pentacel was licensed) and October 31, 2009. Deaths were attributed to SIDS (almost half of the reported cases), congenital/genetic conditions, respiratory infections, positional asphyxia, anoxic encephalopathy, cardiac arrest of undetermined etiology, dilated and hypertrophic cardiomyopathy, two deaths of undetermined cause, one death with no records obtainable, and two deaths with information pending. Dr. Woo stated that the number of cases of SIDS were not concerning and went on to quote the Institute of Medicine (IOM), which had previously concluded that evidence favors rejection of a causal relationship between exposure to multiple vaccines and SIDS.
VAXELIS – injection site pain, swelling, and redness, crying, fever, irritability, decreased appetite, vomiting, somnolence, and death. At this time, post-marketing data on serious adverse events are limited to those events considered to have a causal link to the vaccines containing the antigens of VAXELIS. These include anaphylaxis, hypersensitivity, seizures, including febrile seizures, and excessive swelling of the injected limb. 6 in 1 (hexavalent) vaccines have been in use outside of the United States for several years and numerous studies have linked their use to an increase in infant deaths. In the two U.S. pre-licensing clinical trials, six deaths were reported in infants who received VAXELIS and included sepsis, asphyxia, hydrocephalus, unknown cause, and two cases of sudden infant death syndrome. The number of deaths that occurred during the VAXELIS pre-licensing clinical trials outside of the United States was not provided as part of the vaccine product insert. Pre-licensing clinical trials of VAXELIS compared infants who received VAXELIS concomitantly with RotaTeq (rotavirus vaccine) and Prevnar 13 (13-valent pneumococcal conjugate vaccine) against infants who received Pentacel (DTaP-IPV-HIB), and RECOMBIVAX-HB (hepatitis B, recombinant vaccine) concomitantly with RotaTeq and Prevnar 13. The safety and effectiveness of VAXELIS was evaluated in six clinical trials involving only 5,251 infants between the ages of 43 and 99 days of age at the time of enrollment. Only two clinical trials took place in the United States and involved 3,380 infants between the ages of 46 and 98 days. Information on solicited adverse events included injection site pain, redness, and swelling, fever, irritability, decreased appetite, somnolence, vomiting and crying were recorded by parents for only five days following vaccination. Sixty-eight (2 percent) infants who received VAXELIS were reported to have experienced a serious adverse event within 30 days of vaccination, however, only four of these serious adverse events were attributed to VAXELIS. Three infants experienced serious fever and one infant had a life-threatening event that included vomiting followed by lethargy and pallor. The VAXELIS product insert does not provide any further information on the serious adverse events reported post vaccine administration that were considered by the vaccine manufacturer to be unrelated to the administration of VAXELIS. While VAXELIS is FDA approved for use in infants and children between the ages of six weeks and four years of age, the safety of VAXELIS was only evaluated in infants and children between six weeks and 15 months and the effectiveness of VAXELIS was only evaluated in infants between six weeks and six months of age. Pre-licensing clinical trials did not examine the effectiveness of VAXELIS in infants and children between six months and four years of age or the safety of VAXELIS for use in children between 15 months and four years of age. The FDA granted approval of the vaccine for use in this older population based on pre-licensing clinical trial data involving only younger children. Currently, there are no studies to support the safety of VAXELIS for use in children between the ages of 15 months and four years of age.
Using the MedAlerts search engine, as of March 29, 2024, there have been 93,508 reports of Hib vaccine reactions, hospitalizations, injuries and deaths following Hib vaccinations made to the federal Vaccine Adverse Events Reporting System (VAERS), including 2,927 related deaths, 19,094 hospitalizations, and 1,826 related disabilities. Approximately 85 percent of HIB vaccine-related adverse events occurred in children under three years of age. Of these Hib-vaccine related deaths reported to VAERS,nearly 92 percent of the deaths occurred in children under three years of age. Of these reported deaths, 2,177 occurred in infants under the age of 6 months.
Even though the National Childhood Vaccine Injury Act of 1986 legally required pediatricians and other vaccine providers to report serious health problems following vaccination to federal health agencies (VAERS), many doctors and other medical workers giving vaccines to children and adults fail to report vaccine-related health problem to VAERS. There is evidence that only between one and 10 percent of serious health problems that occur after use of prescription drugs or vaccines in the U.S. are ever reported to federal health officials, who are responsible for regulating the safety of drugs and vaccines and issue national vaccine policy recommendations.
As of April 1, 2024, there had been 179 claims filed in the federal Vaccine Injury Compensation Program (VICP) for injuries and deaths following HIB vaccination, including 34 deaths and 145 serious injuries. Of that number, the U.S. Court of Claims administering the VICP has compensated 61 children and adults who have filed for Hib vaccine injuries.
In the comprehensive report evaluating scientific evidence, Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality, published in 1994 by the Institute of Medicine (IOM), reported vaccine adverse events following administration of Haemophilus influenzae vaccines were evaluated by a physician committee. These adverse events included Guillain-Barre Syndrome, transverse myelitis, thrombocytopenia, early susceptibility to H. influenzae type b, anaphylaxis, and death. In most cases, after evaluating the available literature, the IOM was unable to accept or reject a causal relation between Hib vaccines and the reported side effect due to a lack of evidence. The committee, however, favored a causal association between unconjugated PRP vaccine (unconjugated PRP vaccines are no longer approved for use in the U.S.) and the increased risk of early onset H. influenzae disease in children older than 18 months who received an unconjugated PRP vaccine as their first Hib vaccine. The committee also favored rejection of an association between early onset Hib disease and conjugated Hib vaccines (conjugated Hib vaccines are the only FDA approved Hib vaccines available in the U.S.) Additionally, the IOM favored rejection of a causal relation between death from early-onset Hib disease and conjugated Hib vaccines. Hib vaccines were not evaluated as part of the Institute of Medicine’s 2012 comprehensive report Adverse Effects of Vaccines: Evidence and Causality.
No Hib conjugate or Hib combination vaccines have ever been evaluated for their carcinogenic or mutagenic potential, or for impairment of fertility.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Haemophilus Influenzae Type B (Hib) and the Hib vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.



