Read and report vaccine reactions, harassment and failures.
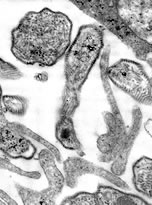
Mumps virus is a contagious paramyxovirus that is comprised of a single-stranded RNA genome. Respiratory secretions transmit the virus and the infection begins in the nasopharynx and regional lymph nodes. After exposure, it generally takes 12 to 25 days for symptoms to develop. These symptoms typically include headache, muscle aches, tiredness, and loss of appetite. During this time, the virus is present in the blood and spreads throughout the body’s tissues. Parotitis, swelling of the parotid gland on one or both sides of the face under the ears and chin, is the most common clinical feature of a mumps infection, and typically occurs within the first two days. Up to 30 percent of people infected with mumps will have no symptoms of infection and up to 50 percent may exhibit signs of a mild nonspecific illness.
Mumps is generally a mild disease in childhood, but it can result in complications, which most often occur in adults. Complications of mumps include inflammation of the testicles in males, inflammation of the breast tissue and ovaries in females, meningitis, encephalitis, and loss of hearing. Fertility problems following mumps infection are rare. Mumps rarely results in death and most people recover from mump infection within a few weeks. Learn more about Mumps…
Mumps Vaccine
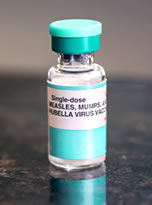
There are three mumps containing vaccines available for use in the United States. Two vaccines, MMRII and PRIORIX , are combination measles-mumps-rubella (MMR) live virus vaccines. The third, ProQuad is a combination measles-mumps-rubella-varicella (MMR-V) live virus vaccine. The CDC recommends that children receive two doses of a mumps containing vaccine, with the first dose between the ages 12-15 months, and the second dose between the ages 4-6 years. The CDC also recommends that individuals born after 1957 and that have no laboratory evidence of immunity or documentation of vaccination should receive at least one dose of MMR vaccine. Two doses of MMR vaccine are also recommended for healthcare personnel, students entering college and other post-high school educational institutions, as well as international travelers, if they have not already been vaccinated as a child.
The CDC also recommends MMR vaccination for infants between 6 and 12 months of age who may be traveling internationally. However, ProQuad, MMRII, and PRIORIX have only been approved for use in the U.S. for children older than 12 months of age. Learn more about Mumps vaccine…
Mumps Quick Facts
Mumps
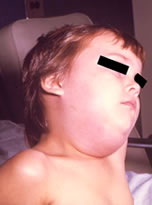
- Mumps is a contagious viral infection and symptoms begin with a headache, muscle aches, tiredness, and loss of appetite . A stiff neck just before, during or after mumps infection is a sign that aseptic meningitis (inflammation of the lining of the brain) may have developed, which is a rare complication of mumps ;
- The typical “signature” physical sign of mumps is visible swelling of one or both sides of the face under the ears and chin. Males, who are past puberty, can experience pain and extreme swelling of the testes and rarely, become sterile. Mumps is very rarely fatal. Continue reading quick facts…
Mumps Vaccine
- There are three mumps containing vaccines available in the U.S. MMRII, manufactured by Merck, and PRIORIX, manufactured by GlaxoSmithKline, contain live attenuated measles, mumps, and rubella virus. ProQuad (MMR-V), also manufactured by Merck, contains live attenuated measles, mumps, rubella, and varicella virus. The CDC recommends that children get two doses of a mumps containing vaccine with the first dose given between ages 12-15 months, and the second dose given between ages 4-6 years;
- Common side effects from the MMR or MMR-V vaccine include low-grade fever, skin rash, itching, hives, swelling, reddening of skin, and weakness. Reported serious adverse reactions following MMR and MMR-V vaccination include seizures, brain inflammation and encephalopathy; thrombocytopenia; joint, muscle and nerve pain; gastrointestinal disorders; measles like rash; conjunctivitis and other serious health problems Continue reading quick facts...
NVIC encourages you to become fully informed about mumps and the mumps vaccine by reading all sections in the Table of Contents below, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
What Is Mumps?
Mumps virus is a contagious paramyxovirus that is comprised of a single-stranded RNA genome. Respiratory secretions transmit the virus and the infection begins in the nasopharynx and regional lymph nodes. After exposure, it generally takes an incubation period of 12 to 25 days for symptoms to develop. These symptoms typically include headache, muscle aches, tiredness, and loss of appetite. During this time, the virus is present in the blood and spreads throughout the body’s tissues. Swelling of the parotid gland (parotitis) on one or both sides of the face under the ears and chin, is the most common clinical feature of a mumps infection, and typically occurs within the first two days. Up to 30 percent of people infected with mumps will have no symptoms of infection (asymptomatic) and up to 50 percent may exhibit signs of a mild nonspecific illness. Mumps is generally a mild disease that most often occurs in childhood; however, it can result in complications, though most complications occur in adults.
Complications of mumps include inflammation of the testicles in males, inflammation of the breast tissue and ovaries in females, meningitis, encephalitis, and loss of hearing. Fertility problems following mumps infection are rare. Mumps rarely results in death and most people recover from mump infection within a few weeks.
A buccal swab conducted within three days of the onset of parotitis is the most preferred method to diagnose a mumps infection. A blood test to confirm the presence of mumps antibodies (IgM) collected soon after the onset of symptoms can also be completed, although mumps IgM may also be present if collected soon after mumps vaccination.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Is Mumps contagious?

The mumps virus is contagious and can be found in the saliva, throat, and urine of an infected person. It is spread through the air by respiratory droplets or by contact with the saliva of an infected person. The mumps virus has been detected from seven days prior to, and up to fourteen days following the onset of swelling of the parotid gland (parotitis) on one or both sides of the face under the ears and chin. However, the highest viral levels generally occur just prior to the onset of parotitis and decrease quickly. It is generally believed that transmission of the virus typically occurs a few days before and after the onset of parotitis. A person infected with mumps can spread the virus to others though:
- talking, coughing, sneezing
- improper hand washing
- sharing utensils or cups
It is also likely that the virus can be transmitted from individuals who are asymptomatic or who show signs of a nonspecific illness.
Frequently, mumps outbreaks occur where many people live in close proximity to one another, such as close-knit communities, prisons, and college campuses. Recently, the majority of mumps outbreaks have occurred in fully vaccinated populations.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
What is the history of Mumps in America and other countries?
The earliest reports of mumps dates back to Hippocrates in the 5th century BC. Hippocrates reported on an illness that involved swelling just below one or both ears and sometimes involved the swelling and pain of one or both testicles. In 1934, researchers determined that the mumps virus that was present in the saliva could be spread from an infected individual to rhesus monkeys. The mumps virus was isolated in 1945 and researchers began work on the development of a vaccine against mumps.
Prior to widespread vaccination programs, mumps outbreaks occurred in the U.S. every 2 to 5 years, mainly among children and in crowded, confined populations such as schools and military bases. In countries where there are no widespread mumps vaccination programs, higher incidences of mumps outbreaks occur every 2 to 5 years, and typically affect children between the age of 5 and 9 years. Mumps outbreaks occur more often in the winter and spring however outbreaks are possible at any time of the year.
Mumps was considered a nationally reportable infection between 1922 and 1950; however, it was removed from the list in 1951. In 1968, one year after the introduction of Mumpsvax, a live virus vaccine manufactured by Merck, the CDC resumed data collection on mumps infections. In 1968, there were 152,209 reported cases in the United States. Reported cases of mumps continued to decrease and by 1977, there were only 21,436 cases reported in the United States. By 1985, the number of reported cases had decreased further with only 2,982 cases of mumps reported to the CDC. However, between 1985 and 1987, a resurgence of mumps occurred and by 1987, the number of reported cases had risen to 12,848. The demographics of mumps infection shifted during this resurgence, with nearly one third of cases occurring in individuals 15 years of age and older, a demographic with an increased risk of complications. Prior to 1985, mumps infections predominantly affected children between the age of 5 and 9 years of age.
After 1987, mumps infections continued to decrease again and by 1989, when the CDC’s Advisory Committee on Immunization Practices (ACIP) voted to recommend two doses of the MMR vaccine due to the resurgence of measles in the United States, only 666 cases of mumps were reported to the CDC.
However, in late 2004, an outbreak of mumps disease occurred in the United Kingdom and by late 2005, 56,390 cases of mumps had been reported, with the majority occurring in persons aged 15 to 24 years of age, most of whom had not previously been vaccinated for mumps.
In the U.S., infections continued to decline until December of 2005, when a large outbreak began at an eastern Iowa university. The outbreak continued to spread, affecting many fully vaccinated college students, and by the end of 2006, 6,584 cases of mumps infection had been reported to the CDC.
In 2009 and 2010, the U.S., Canada, and Guam experienced mumps outbreaks. In the U.S., the majority of cases occurred in the Northeast, affecting mainly adolescent Orthodox Jewish boys. There were 4,603 cases of mumps reported to the CDC as a result of the outbreaks that occurred in 2009 and 2010. As with the 2006 outbreak, most persons affected with mumps were previously vaccinated for mumps.
Outbreaks in the United States have continued to occur in highly vaccinated populations, especially among young adults residing on college campuses. In 2011, an outbreak occurred on a university campus in California. In 2015-2016, several Midwest universities experienced outbreaks, again, affecting highly vaccinated students. In 2016-2017, nearly 3000 people living in a close-knit community in Northwest Arkansas were infected with mumps. In October 2017, as a result of the continued outbreaks among highly vaccinated individuals, the CDC’s ACIP recommended that a third dose of a mumps containing vaccine be administered in the event of an outbreak of the illness.
Mumps outbreaks continue to occur in the United States and between January 1 and December 28, 2019, there were 3,474 cases of mumps reported to the CDC. As up to 30 percent of people infected with mumps infection are asymptomatic, and up to 50 percent may exhibit signs of a mild nonspecific illness, it is likely that mumps infection rates are significantly higher than the number of reported cases.
From April 1 to December 31, 2020, 142 cases of mumps were reported in the U.S., a decrease from the previous six years. Health officials have speculated that preventative measures to stop the spread of COVID-19 illness may have also led to a decrease in mumps cases. In 2021, there were 154 reported mumps cases.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Can Mumps Cause Injury and Death?

Complications and death from mumps infections are rare. Complications include inflammation of the testicles in males, which can, in rare cases, result in atrophied testicles and lead to sterility.
Aseptic meningitis may also develop as a complication of mumps. Rarely, mumps may also cause pancreatitis, encephalitis, oophoritis (inflamed ovaries), hearing loss, mastitis, myocarditis, thyroiditis, nephritis, arthritis, diabetes, and thrombocytopenic purpura. Spontaneous abortions (miscarriage) in pregnant women infected with mumps have also been reported .
No mumps related deaths have been reported in recent years.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Who is at highest risk for getting Mumps?

Prior to the recommendation of routine mumps vaccination, mumps infections most commonly affected children between the ages of 5 and 9 years of age. However, since the mid 1980’s, the demographic of mumps infection in the U.S. has shifted, resulting in a higher risk of mumps infection in older children, adolescents, and young adults. People who travel to high-risk countries where mumps is endemic and those who spend a great deal of time in crowded, confined settings such as daycare centers, schools, college dormitories and military bases, are also at higher risk.
Individuals with compromised immune systems, such as those infected with HIV/AIDS, undergoing chemotherapy treatment for cancer, and those taking oral steroids, may also be at higher risk for contracting mumps infection.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Who is at highest risk for suffering complications from Mumps?

Adults are more likely to suffer complications from mumps infection. Pregnant women may also be at a higher risk of miscarriage should infection occur early in pregnancy.
Complications from mumps infection are rare but can lead to hearing loss, pancreatitis, swelling of the ovaries in women, swelling of the testicles in post-pubertal males, meningitis and encephalitis.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Can Mumps be prevented and are there treatment options?

Prevention of mumps involves reducing the transmission of infections. This includes:
- not sharing eating utensils or drink containers;
- washing hands often with soap and water;
- cleaning surfaces frequently handled by others, such as toys, doorknobs, tables, and counters;
- staying home if sick;
- covering the mouth and nose with a tissue when coughing or sneezing and disposing of the tissue immediately.
There is no specific treatment for mumps except alleviation of symptoms with rest, hydration, soft diet, pain relievers, cool compresses and avoidance of acidic foods and beverages.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
What is Mumps vaccine?
There are three mumps containing vaccines available for use in the United States. Two vaccines, MMRII, manufactured by Merck, and PRIORIX, manufactured by GlaxoSmithKline, are combination measles-mumps-rubella (MMR) live virus vaccines. The third, ProQuad, manufactured by Merck, is a combination measles-mumps-rubella-varicella (MMR-V) live virus vaccine. Package inserts for mumps vaccine can be found on NVIC's Mumps Quick Facts page.
MMRII is licensed and recommended in the U.S. for individuals aged 12 months or older. It is a live attenuated virus vaccine propagated in chick embryo cells and cultured with Jeryl Lynn live attenuated virus mumps and Meruvax II, a live attenuated virus vaccine developed using WI-38 human diploid lung fibroblasts. The WI-38 human diploid cell line was derived from the lung tissue of a three-month human female embryo. The growth medium used was salt solution and 10 percent calf (bovine) serum.
ProQuad is licensed and recommended in the U.S. for individuals aged 12 months to 12 years of age. ProQuad (Measles, Mumps, Rubella and Varicella Virus Vaccine Live) is a combined, attenuated, live virus vaccine containing measles, mumps, rubella, and varicella viruses. ProQuad is a sterile lyophilized preparation of the components of M-M-R II (Measles, Mumps, and Rubella Virus Vaccine Live): Measles Virus Vaccine Live, and Varicella Virus Vaccine Live (Oka/Merck), the Oka/Merck strain of varicella-zoster virus developed using MRC-5 cells. MRC-5 cells are derived from a cell line that was developed in 1966 from lung tissue taken from a 14 week aborted fetus and contains viral antigens.
The growth medium for measles and mumps for both MMRII and ProQuad is a buffered salt solution containing vitamins and amino acids and supplemented with fetal bovine serum containing sucrose, phosphate, glutamate, and recombinant human albumin, and neomycin.
The growth medium for rubella is a buffered salt solution containing vitamins and amino acids and supplemented with fetal bovine serum containing recombinant human albumin and neomycin. Sorbitol and hydrolyzed gelatin stabilizer are added to the individual virus harvests. In the ProQuad vaccine, the Oka/Merck strain of the live attenuated varicella virus, initially obtained from a child with wild-type varicella, then introduced into human embryonic lung cell cultures, adapted to and propagated in embryonic guinea pig cell cultures and finally developed using human diploid cell cultures (WI-38) is added to the MMRII component.
According to Merck, both MMRII and ProQuad vaccines are screened for microorganisms that may have been unintentionally introduced in the manufacturing process (adventitious agents ). Each dose of MMRII contains sorbitol, sodium phosphate, sucrose, sodium chloride, hydrolyzed gelatin, recombinant human albumin, fetal bovine serum, other buffer and media ingredients and neomycin. Each dose of ProQuad contains sucrose, hydrolyzed gelatin, sorbitol, MSG, sodium phosphate, human albumin, sodium bicarbonate, potassium phosphate and chloride, neomycin, bovine calf serum, chick embryo cell culture, WI-38 human diploid lung fibroblasts and MRC-5 cells.
The MMRII vaccine product information insert states that the MMRII vaccine should be given one month before or one month after any other live viral vaccines. The ProQuad vaccine product information insert states that one month should lapse between administration of ProQuad and another measles containing vaccine such as MMRII and at least three months should lapse between ProQuad and any varicella containing vaccine.
PRIORIX is licensed and recommended for individuals aged 12 months or older. PRIORIX is made up of the Schwarz strain of live attenuated measles virus and the RIT 4385 strain of live attenuated mumps virus, derived from the Jeryl Lynn mumps strain, both propagated in chick-embryo fibroblasts. This vaccine was also developed using the Wistar RA 27/3 strain of live attenuated rubella virus propagated in MRC-5 human diploid cells.
These three virus strains are cultured in media containing amino acids, neomycin sulfate and bovine serum albumin. Multiple washings are done to remove the antibiotic and albumin from the media. The attenuated measles, mumps and rubella viruses are then mixed with a stabilizer before lyophilization. After reconstitution, the vaccine is a clear peach- to fuchsia pink-colored suspension. In addition to the measles, mumps, and rubella viruses, each 0.5ml dose also contains amino acids, mannitol, anhydrous lactose, and sorbitol. Each dose may also contain residual amounts of ovalbumin, bovine serum albumin and neomycin sulphate.
The tip caps of the prefilled syringes of diluent for PRIORIX contain natural rubber latex.
The CDC recommends that children receive two doses of a mumps containing vaccine, with the first dose between the ages 12-15 months, and the second dose between the ages 4-6 years. The CDC also recommends that individuals born after 1957 and have no laboratory evidence of immunity or documentation of vaccination should receive at least one dose of MMR vaccine. Two doses of MMR vaccine are also recommended for healthcare personnel, students entering college and other post-high school educational institutions, as well as international travelers.
The CDC also recommends MMR vaccination for infants between 6 and 12 months of age who may be traveling internationally. However, ProQuad, and MMRII have only been approved for use in for children older than 12 months of age. The MMRII vaccine product insert states that effectiveness and safety of administration of MMRII has not been established in children between the ages of 6 and 12 months of age and if administered to this population, antibodies may not develop. According to the CDC, an infant vaccinated prior to 12 months of age would still require two additional doses of MMR vaccine.
In October of 2017, following numerous outbreaks of mumps infections throughout the United States, most notably on college campuses, the CDC recommended a third dose of a mumps containing vaccine be administered in the event of an outbreak.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
What is the history of Mumps vaccine use in America?

The mumps virus was detected in 1934, and isolated in 1945; however it took researchers until 1948 to grow the virus in a laboratory setting. The first mumps vaccine, a killed virus vaccine, was developed for use in the United States in 1948. This vaccine, producing only short-term immunity, was available and used from 1950 until its discontinuation in 1978.
In 1963, vaccine researcher Maurice Hilleman used samples from his own daughter’s mumps case to isolate the mumps virus. This mumps strain, known as the Jeryl Lyn Strain named for his daughter, was used to create Mumpsvax, the first live mumps virus vaccine. Mumpsvax, manufactured by Merck, became available for use in the United States in 1967. In 1971, Mumpsvax was combined with the measles and rubella vaccine to become the MMR vaccine. Currently, mumps vaccine is only available in combination with measles and rubella (MMRII) and measles, rubella, and varicella (ProQuad). Both vaccines are manufactured by Merck.
In 1977, the CDC adopted its Advisory Committee on Immunization Practices’ (ACIP) recommendation for a single dose of mumps vaccine for all children at 12 months of age. However, in response to a resurgence of measles in the United States in 1989, the CDC updated its recommendation, recommending that two doses of a measles containing vaccine, preferably the MMR vaccine, be administered to all children. Recommendations were updated again in 1998 when the CDC recommended that the MMR vaccine be the vaccine of choice and for all fifty states to adopt vaccine legislation requiring that children receive two doses of MMR vaccine, after the age of 12 months, and at least one month apart, for school entry. At the time of this recommendation, the CDC’s ACIP did not consider the second dose of MMR vaccine to be a booster dose for mumps or rubella, reporting “a primary immune response to the first dose provides long-term protection.” They did, however, report that field studies on the mumps vaccine indicated an estimated vaccine effectiveness to be between 75 and 95 percent.
Mumps infections in the U.S. remained low until late 2005, when the Midwest experienced a large outbreak in a highly vaccinated population that included several college campuses. As a result of this outbreak, the CDC adopted the ACIP’s recommendation for two doses of mumps vaccine for school aged children and high-risk adults in May 2006. High-risk adults were defined as college students, health care providers, and international travelers. Mumps outbreaks continued to occur both in the United States as well as abroad.
In 2010, two former Merck employees filed a lawsuit alleging that Merck altered testing and study results to make the mumps vaccine appear more effective than it is in preventing mumps in children. The lawsuit, unsealed in 2012, also claimed that outbreaks in vaccinated populations were directly related to the falsification of the mumps efficacy data.
“Specifically, the suit claims Merck manipulated the results of clinical trials beginning in the late 1990s so as to be able to report that the combined mumps vaccine, known as MMR-II (a revised version of the 1971 MMR shot containing a different strain of the rubella virus), is 95 percent effective, in an effort to maintain its exclusive license to manufacture it. This percentage is the benchmark used by the FDA to grant Merck approval to sell its original mumps vaccine in 1967.”
Scientists involved in the whistleblower case claimed that Merck falsified vaccine efficacy testing by adding animal antibodies to the samples in order to demonstrate a vaccine effectiveness of 95 percent.
Merck denied all charges in connection with the lawsuit and stated:
"Merck has presented information that demonstrated to the United States Department of Justice that these allegations are factually false and after the department conducted its own two-year investigation, it decided not to pursue this lawsuit."
In 2015, Merck was accused by attorneys representing the scientists of stonewalling the case by stating that they are unable to perform current clinical trials of the mumps vaccine to determine current efficacy of the vaccine and instead provided the court with 50-year old efficacy data. Trial proceedings in the case were expected to begin in 2018, however, was delayed until late 2019.
In 2017, as a result of continued outbreaks in fully vaccinated populations, the CDC adopted the ACIP’s recommendation that a third dose of a mumps containing vaccine be administered in the event of an outbreak, stating:
“Current routine recommendation for 2 doses of MMR vaccine appears to be sufficient for mumps control in the general population, but insufficient for preventing mumps outbreaks in prolonged, close-contact settings, even where coverage with 2 doses of MMR vaccine is high.”
In June 2022, the FDA approved PRIORIX, a live attenuated measles, mumps, and rubella vaccine, manufactured by GlaxoSmithKline. PRIORIX was initially licensed in Germany in 1997 and according to the CDC, the vaccine has been in use globally in nearly 100 countries. On June 23, 2022, the CDC’s ACIP voted to approve use of PRIORIX as an option for the MMR vaccine according to the current MMR recommendations and off-label uses.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
How effective Is Mumps vaccine?

According to the manufacturer’s product insert, the mumps vaccine is 96 percent effective. However, the Centers for Disease Control (CDC) reports that two doses of mumps vaccine are between 31 and 95 percent effective, while a single dose is 49 to 91 percent effective.
Mumps outbreaks in highly vaccinated populations began in 2006 with the majority of cases occurring among young adults between the ages of 18 and 24. By the late 2000s, researchers began speculating that the lack of natural boosting from exposure to wild-type mumps may be resulting in waning of vaccine acquired immunity. Moreover, the number of asymptomatic patients transmitting the infection to others may be higher than the estimated 30 percent, thus affecting public health measures designed to contain the outbreak. As a result of the resurgence in mumps cases among highly vaccinated individuals, experts reported that measures to locate unvaccinated individuals would not be helpful, as they were not considered to be responsible for mumps outbreaks. A third dose of mumps vaccine (MMR) was suggested as method of preventing and containing further outbreaks.
Studies have noted mumps vaccine waning as evidenced by outbreaks occurring more frequently among adults, rather than children, with researchers predicting an increase in mumps outbreaks as the temporary vaccine-induced immunity replaces longer lasting natural immunity to the disease. The time between the last dose of mumps vaccine (MMR) and the onset of the disease appears to be a factor in outbreaks, suggestive of vaccine waning and its inability to confer long lasting immunity.
Several researchers have reported the current two-dose MMR vaccine strategy to be ineffective at preventing mumps outbreaks. Additionally, while health officials believe that the administration of a third dose of MMR vaccine may assist in controlling an outbreak, they have also indicated that routine recommendation of an additional dose will not prevent mumps outbreaks. Both the rapid decrease in vaccine induced mumps antibody levels, as well as the emergence of mumps strains not targeted by the vaccine, may cause additional booster doses of mumps vaccine to be ineffective at preventing and controlling mumps outbreaks. The limited effectiveness of the current vaccine strategies to prevent mumps outbreaks has prompted several experts to recommend that more research be dedicated to examining the immune system’s response to mumps vaccination. Numerous studies focused on the continued mumps outbreaks occurring in highly vaccinated populations have many researchers suggesting that both the waning of vaccine induced immunity and the lack of an effective mumps vaccine may be to blame.
The MMRII and ProQuad (MMRV) vaccines contain mumps genotype A, the Jeryl Lynn strain, isolated from samples collected in 1963. However, since 2006, mumps genotype G has become the predominant circulating strain of mumps in the United States. The CDC reports that while studies have found the Jeryl Lynn (genotype A) strain effective at preventing mumps infections caused by genotype G, vaccine induced antibodies have been noted to be lower. In 2015-2016, a large mumps outbreak involving mumps genotype G in Norway concluded that the genotype A found in the MMR vaccine offered “suboptimal protection against mumps genotype G”. Additional studies have also reported that the Jeryl Lynn mumps strain to be inadequate to protect against the strains of mumps that are currently circulating.
In 2010, two former Merck employees filed a lawsuit alleging that Merck altered the vaccine efficacy testing and study results in an attempt to make the mumps vaccine appear more effective than it is. Specifically, the lawsuit made claims that mumps outbreaks in vaccinated individuals were directly related to the alleged falsification of efficacy data. As of December 4, 2022, the lawsuit was still pending.
In October of 2017, as a result of continual mumps outbreaks in highly vaccinated populations, the CDC’s Advisory Committee on Immunization Practices (ACIP) recommended a third dose of mumps vaccine (MMR) to be administered in the event of an outbreak. At the time of this recommendation, the use of a third MMR vaccine was reported to be between 61 and 88 percent effective at preventing mumps infection.
In June 2022, the FDA and CDC approved use of PRIORIX, a live attenuated measles, mumps, and rubella vaccine, for individuals 12 months of age and old. The effectiveness of PRIORIX was based on antibody responses when compared to the MMRII vaccine and according to the package insert, PRIORIX was considered non-inferior to Merck’s MMRII vaccine.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Can Mumps Vaccine Cause Injury & Death?

The Centers for Disease Control (CDC) report minor side effects from the MMR-V and MMR vaccines to include fever, injection site redness or rash, pain at the injection site, facial or neck swelling, pneumonia, full body rash, swelling of the brain and/or spinal cord covering, temporary low platelet count, temporary stiffness and pain at the joints, and seizures. Allergic reactions, serious injury, and death can also occur after vaccination. A vaccine strain infection following vaccination may be the cause of the full body rash.
There is a significantly greater risk of seizures following MMR-V vaccine in comparison to separate administrations of MMR and varicella vaccines if the MMR-V is given as the first dose of the series.
Persons with serious immune disorders are at risk of developing a life-threatening infection if administered the MMR-V or MMR vaccine. Vaccination is not recommended in this population.
Serious complications reported by Merck in the MMRII package insert during vaccine post-marketing surveillance include:
- brain inflammation (encephalitis) and encephalopathy (chronic brain dysfunction);
- panniculitis (inflammation of the fat layer under the skin);
- atypical measles;
- syncope (sudden loss of consciousness, fainting);
- vasculitis (inflammation of the blood vessels);
- pancreatitis (inflammation of the pancreas);
- diabetes mellitus;
- thrombocytopenia purpura (blood disorder);
- Henoch-Schönlein purpura (inflammation and bleeding in the small blood vessels);
- acute hemorrhagic edema of infancy (rare vasculitis of the skin’s small vessels occurring in infants);
- leukocytosis (high white blood cell count);
- anaphylaxis (shock);
- bronchial spasms;
- pneumonia;
- pneumonitis (inflammation of the lung tissues);
- arthritis and arthralgia (joint pain);
- myalgia (muscle pain);
- polyneuritis (inflammation of several nerves simultaneously);
- measles inclusion body encephalitis (disease affecting the brain of immunocompromised persons);
- subacute sclerosing panencephalitis (fatal progressive brain disorder caused by exposure to the measles virus);
- Guillain-Barre Syndrome (GBS)(disease where the body’s immune system attacks the nerves);
- acute disseminated encephalomyelitis (ADEM) (brief widespread inflammation of the nerve’s protective covering);
- transverse myelitis (inflammation of the spinal cord);
- aseptic meningitis;
- erythema multiforme (skin disorder from an allergic reaction or infection);
- urticarial rash (hives, itching from an allergic reaction);
- measles-like rash;
- Stevens-Johnson syndrome (severe reaction causing the skin and mucous membranes to blister, die, and shed);
- nerve deafness (hearing loss from damage to the inner ear);
- otitis media (ear infection);
- retinitis (inflammation of the retina of the eye);
- optic neuritis (inflammation of the optic nerve);
- conjunctivitis (pink eye);
- ocular palsies (dysfunction of the ocular nerve);
- epididymitis (inflammation of the epididymis);
- paresthesia (burning or prickling of the skin);
- death.
Serious complications reported by Merck in the ProQuad package insert during vaccine post-marketing surveillance include:
- measles;
- atypical measles;
- vaccine strain varicella;
- varicella-like rash;
- herpes zoster;
- herpes simplex;
- pneumonia and respiratory infection;
- pneumonitis;
- bronchitis;
- epididymitis;
- cellulitis;
- skin infection;
- subacute sclerosing panencephalitis;
- aseptic meningitis;
- thrombocytopenia;
- aplastic anemia (anemia due to the bone marrow’s inability to produce platelets, red and white blood cells);
- lymphadenitis (inflammation of the lymph nodes);
- anaphylaxis including related symptoms of peripheral, angioneurotic and facial edema;
- agitation;
- ocular palsies;
- necrotizing retinitis (inflammation of the eye);
- nerve deafness;
- optic and retrobulbar neuritis (inflammation of the optic nerve);
- Bell’s palsy (sudden but temporary weakness of one half of the face);
- cerebrovascular accident (stroke);
- acute disseminated encephalomyelitis;
- measles inclusion body encephalitis;
- transverse myelitis;
- encephalopathy;
- Guillain-Barré syndrome;
- syncope (fainting);
- tremor;
- dizziness;
- paraesthesia;
- febrile seizure;
- afebrile seizures or convulsions;
- polyneuropathy (dysfunction of numerous peripheral nerves of the body);
- Stevens-Johnson syndrome;
- Henoch-Schönlein purpura;
- acute hemorrhagic edema of infancy;
- erythema multiforme;
- panniculitis;
- arthritis;
Serious complications reported by GlaxoSmithKline in the PRIORIX package insert during vaccine post-marketing surveillance have included:
- Vasculitis (including Henoch-Schönlein purpura and Kawasaki syndrome);
- Thrombocytopenia and thrombocytopenic purpura;
- Anaphylactic reactions;
- Meningitis;
- “Mumps like” illness;
- “Measles like” illness;
- Orchitis;
- Epididymitis;
- Parotitis;
- Erythema multiforme;
- Arthralgia;
- Arthritis;
- Encephalitis;
- Cerebellitis;
- Cerebellitis-like symptoms (including transient gait disturbance and transient ataxia);
- Guillain-Barré syndrome;
- Transverse myelitis;
- Peripheral neuritis;
- Afebrile seizures;
A 2014 published study on the MMR-V vaccine in Canada determined that the risk of febrile seizures to be double in children receiving the MMR-V vaccine when compared to those receiving the MMR and varicella vaccine separately. A 2015 meta-analysis concluded a two-fold increase in febrile seizures between 5 and 12 days or 7 and 10 days following MMR-V vaccination in children between the ages of 10 and 24 months.
ProQuad vaccine contains albumin, a human blood derivative and as a result, a theoretical risk of contamination with Creutzfeldt-Jakob disease (CJD) exists. Merck states that no cases of transmission of CJD or other viral diseases have been identified and all virus pools, cells, bovine serum, and human albumin used in vaccine manufacturing are all tested to assure the final product is free of potentially harmful agents.
The MMRII and the ProQuad product inserts report that cases of measles inclusion body encephalitis, pneumonitis and death have occurred in severely immunocompromised individuals who were inadvertently vaccinated and disseminated mumps and rubella infections have also been reported in this population.
In the comprehensive report evaluating scientific evidence, Adverse Effects of Vaccines: Evidence and Causality , published in 2012 by the Institute of Medicine (IOM), 30 reported vaccine adverse events following the Measles, Mumps, and Rubella (MMR) vaccine were evaluated by a physician committee . These adverse events included measles inclusion body encephalitis, febrile seizures, arthritis, meningitis, Guillain Barre Syndrome, autism, diabetes mellitus, optic neuritis, transverse myelitis and more.
In 23 of the 30 measles, mumps, and rubella (MMR) vaccine-related adverse events evaluated, the IOM committee concluded that there was inadequate evidence to support or reject a causal relationship between the MMR vaccine and the reported adverse event, primarily because there was either an absence of methodologically sound published studies or too few quality studies to make a determination. The IOM committee, however, concluded that the scientific evidence “convincingly supports” a causal relationship between febrile seizures, anaphylaxis, and measles inclusion body encephalitis in immunocompromised individuals and the MMR vaccine and favored acceptance of a causal relationship between transient arthralgia in both children and women and the MMR vaccine. The IOM committee also concluded that it favored rejection of a causal association between both autism and the MMR vaccine and Type 1 diabetes and the MMR vaccine, however, both of these conclusions resulted following the review of only five epidemiological studies.
A systematic review published in 2012 by the Cochrane Collaboration examined 57 studies and clinical trials involving approximately 14.7 million children who had received the MMR vaccine. While the study authors said they were not able to detect a “significant” association between MMR vaccine and autism, asthma, leukemia, hay fever, type I diabetes, gait disturbance, Crohn’s disease, demyelinating diseases or bacterial or viral infections, they added that:
“The design and reporting of safety outcomes in MMR vaccine studies, both pre- and post-marketing, are largely inadequate.”
In an updated review published in April of 2020, the Cochrane Collaboration reviewed 87 safety studies associated with MMR, MMR-V, and MMR + Varicella vaccine. This review concluded that there was an association between MMR vaccines containing Leningrad-Zagreb and Urabe mumps strains and aseptic meningitis, but no evidence to support this association for MMR vaccines which contain the Jeryl Lynn mumps strains. This conclusion was based on the evaluation of nine studies, all of which were considered low certainty studies. The Cochrane Collaboration reports low certainty studies to be those where their confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
An association was also found between MMR vaccines and idiopathic thrombocytopenic purpura (ITP) and MMR, MMR-V, and MMR + Varicella vaccines and febrile seizures and the studies evaluated to make this determination were a combination of both moderate certainty and low certainty studies. Moderate certainty studies are those in which the Cochrane Collaboration are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
.
The Cochrane Collaboration reported that no association was found between MMR vaccine and encephalitis, encephalopathy, cognitive delay, type 1 diabetes, asthma, dermatitis/eczema, hay fever, leukemia, multiple sclerosis, gait disturbance, and bacterial or viral infections; however, all the studies that were evaluated were found to be either low certainty or very low certainty studies. Very low certainty studies are those in which the Collaboration have very little confidence in the effect estimate
and that the true effect is likely to be substantially different from the estimate of effect
.
No association was found between autism spectrum disorders and MMR vaccines, and the studies evaluated were a combination of moderate certainty and low certainty studies. The Cochrane Collaboration also reported that there was insufficient evidence to support or reject an association between MMR vaccines and inflammatory bowel disease.
Published studies have shown that the MMR vaccine components or excipients, particularly egg antigens and porcine or bovine gelatin, can trigger both immediate and delayed anaphylactic reactions.
In November 2014, the National Vaccine Information Center published a special report The Emerging Risks of Live Virus and Virus Vectored Vaccines: Vaccine Strain Virus Infection, Shedding and Transmission.28 This report reviewed the medical literature for evidence that live virus vaccine strain infection, shedding and potential for transmission occurs, including mumps vaccine strain infection and shedding.
In 2006, a published report confirmed the transmission of Leningrad-3 live attenuated mumps vaccine virus infection from healthy vaccinated children in Russia to close contacts of previously vaccinated children. The six vaccinated children had mumps symptoms, but the 13 close contacts did not have symptoms even though some of them tested positive for mumps vaccine strain infection.
In 2008, a published report confirmed the transmission of L-Zagreb mumps vaccine strain virus infection and transmission by three vaccinated children in Croatia to five adult parent contacts. Mumps symptoms began in the children within three weeks of vaccination and symptoms began in the parents within five to seven weeks after the children were vaccinated. One of the affected adults suffered mumps vaccine strain associated aseptic meningitis.
Merck’s ProQuad vaccine product insert reports that transmission of varicella vaccine virus may occur between vaccine recipients and susceptible contacts, including high risk individuals resulting in both the development or non-development of varicella-like rash. As a result, Merck cautions that vaccine recipients should attempt to avoid close contact with high-risk individuals. This high-risk population includes pregnant women who lack a positive history of illness or vaccination and their newborn infants, any infants born prior to 28 weeks gestation, and all immunocompromised individuals.
Both wild-type mumps and the live Urabe mumps vaccine strain are causally associated with aseptic meningitis (inflammation of the brain), a mumps virus infection complication. The MMRII and ProQuad vaccines contain the Jeryl Lynn mumps vaccine strain and both product inserts deny that this particular strain can cause aseptic meningitis. PRIORIX contains the RIT 4385 strain of live attenuated mumps virus, which is derived from the Jeryl Lynn strain. Though PRIORIX is derived from the Jeryl Lynn strain, health officials state there is no causal link to aseptic meningitis.
As of March 29, 2024, there have been 478 deaths reported to VAERS in association with the MMR vaccine and 40 deaths associated with the MMR-V vaccine. However, the numbers of vaccine-related injuries and deaths reported to VAERS may not reflect the true number of serious health problems that occur develop after MMR vaccination.
Even though the National Childhood Vaccine Injury Act of 1986 legally required pediatricians and other vaccine providers to report serious health problems following vaccination to federal health agencies (VAERS), many doctors and other medical workers giving vaccines to children and adults fail to report vaccine-related health problem to VAERS. There is evidence that only between one and 10 percent of serious health problems that occur after use of prescription drugs or vaccines in the U.S. are ever reported to federal health officials, who are responsible for regulating the safety of drugs and vaccines and issue national vaccine policy recommendations.
As of April 1, 2024, there have been 1,198 claims filed so far in the federal Vaccine Injury Compensation Program (VICP) for 66 deaths and 1,132 injuries that occurred after vaccination with a mumps containing vaccine (MMR, MMR-V, mumps). Of that number, the U.S. Court of Claims administering the VICP has compensated 464 children and adults, who have filed mumps vaccine injury.
One example of an MMR vaccine injury claim awarded compensation in the VICP is the case of O.R. On February 13, 2013, O.R. received the MMR, Haemophilus Influenzae type B, Pneumonia (Prevnar 13), Hepatitis A, and Varicella vaccines. That evening, following vaccination, she became feverish and irritable prompting her mother to contact the doctor. The doctor advised O.R.’s mom to administer Benadryl and Tylenol for her symptoms. The fever persisted for several days and was followed by a severe seizure resulting in cardiac and respiratory arrest. The cardiac arrest and seizures caused O.R. to develop encephalopathy, kidney failure, severe brain injury, low muscle tone and cortical vision impairment. After several months of inpatient hospitalization, O.R. was discharged home with 24-hour supervised medical care. On November 20, 2017, the court conceded that the MMR vaccine caused her encephalopathy and O.R. was awarded a $101 million dollar settlement to cover medical expenses for the rest of her life.
For more information on reported MMR vaccine risks, adverse events and contraindications, see Measles and Measles Vaccine here.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Who is at highest risk for complications from Mumps vaccine?

According to the MMRII product insert, persons most at risk for complications from MMRII vaccine include individuals with both primary and acquired immunodeficiency such as AIDS, dysgammaglobulinemic and hypogammaglobulinemic states, and cellular immune deficiencies. Pneumonitis, measles inclusion body encephalitis, and death have also occurred as a result of being inadvertently vaccinated with a measles containing vaccine.
Persons with thrombocytopenia or history of the condition may also be at greater risk for exacerbation or redevelopment of thrombocytopenia with subsequent doses of MMRII vaccine.
Individuals with a personal history of cerebral injury, personal or family history of seizures, or any other health condition where stress related to fever should be avoided, may also be at greater risk for complications.
As both the live measles and live mumps vaccines are manufactured using chick embryo cell culture, individuals with a history of an immediate reaction, as well as those with anaphylactic and anaphylactoid reactions to eggs may be at greater risk of a reaction from the MMRII vaccine. MMRII contains neomycin and persons who have previously experienced an anaphylactic reaction to either systematic or topical neomycin should not be vaccinated with MMRII due to the risk of reaction and subsequent complications resulting from the reaction.
Merck’s ProQuad (MMR-V) vaccine product insert states that children between the ages of 12 and 23 months with no history of vaccination or wild-type infection with measles, mumps, rubella, and varicella have a higher risk of fever and febrile seizure between 5 and 12 days following vaccination with ProQuad in comparison with children who were vaccinated with separate doses of MMRII and Varicella vaccine. Children with a personal or family history of convulsion or a personal history of cerebral illness or medical condition where stress from fever should be avoided may also be at a greater risk of complications from ProQuad.
Individuals most at risk for complications from ProQuad vaccine include persons with both primary and acquired immunodeficiency such as AIDS, dysgammaglobulinemic and hypogammaglobulinemic states, and cellular immune deficiencies. Pneumonitis, measles inclusion body encephalitis, and death have also occurred as a result of being inadvertently vaccinated with a measles containing vaccine. As well, reports of disseminated varicella vaccine virus infection occurring in children with underlying immunodeficiency disorders inadvertently with a varicella-containing vaccine have also been documented.
According to the package insert for GlaxoSmithKline’s PRIORIX, individuals most at risk for complications from vaccination include persons with a past history of allergic or anaphylaxis to any ingredient of the vaccine, or to a previous dose of the vaccine. Additionally, pregnant women and individuals who are immunosuppressed who are vaccinated with PRIORIX are at a high risk of suffering complications.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Who should not get Mumps vaccine?

Contraindications to receiving the MMRII vaccine documented in Merck’s product insert include:
- Persons who have experienced a severe allergic reaction or anaphylaxis to any MMR vaccine component, including gelatin and neomycin, should not be vaccinated with MMR.
- Pregnant women should not receive this vaccine, as well as women seeking to become pregnant should avoid become pregnant for 3 months following MMR vaccination.
- Individuals receiving immunosuppressive therapy. Vaccination with MMR should be delayed for 3 months following the administration of human immune globulin, blood, or plasma.
- Persons with leukemia, lymphoma, blood dyscrasias and other malignant neoplasms affecting the lymphatic systems or bone marrow.
- Individuals with febrile respiratory illness or other active febrile infection should avoid MMR vaccine.
- MMR and other measles-containing vaccines are not recommended for HIV-infected persons with evidence of severe immunosuppression.
- Persons with a family history of hereditary or congenital immunodeficiency should not be vaccinated with MMR until the immune competence of the recipient has been determined.
- Individuals with untreated tuberculosis should not be vaccinated with MMR vaccine.
Merck’s MMRII product insert also warns that caution should be taken when administering the vaccine to individuals with a history of cerebral injury, family or personal history of convulsions, or any other condition where stress related to fever should be avoided. As well, a person with thrombocytopenia may exacerbate their condition by receiving the MMR vaccine.
Both live measles and mumps vaccine are manufactured in chick embryo cell culture. Extreme caution should be taken when vaccinating individuals with a history of anaphylaxis or immediate hypersensitivity to eggs and Merck advises careful evaluation of the risks and benefits when considering vaccination in this population.
Rubella vaccine virus has been found in the breast milk of nursing mothers with documentation of its ability to be transferred to infants. Serological evidence of rubella infection and a case of mild clinical illness typical with an acquired rubella infection has also been documented in a nursing infant. As a result of these findings, Merck cautions the use of MMR vaccine in nursing women.
IMPORTANT NOTE: Even though the CDC’s Advisory Committee on Immunization Practices (ACIP) states that Merck’s MMRII vaccine can be administer at the same time as other viral and bacterial vaccines, Merck’s MMRII product information insert states that other live virus vaccines—such as varicella should NOT be given at the same time as MMR vaccine but rather should be administered one month prior or one month after MMR vaccination.
Additionally, Merck’s product insert does not recommend giving MMRII at the same time as DTP (diphtheria, tetanus, pertussis) and/or OPV (oral poliovirus vaccine) even though the Advisory Committee on Immunization Practices (ACIP) has stated that simultaneous administration of the entire recommended vaccine series is acceptable.
MMRII vaccine is approved for use in persons 12 months of age and older. Despite recommendations by the CDC’s ACIP that children between 6 and 12 months who will be traveling or residing abroad be vaccinated with MMR prior to international travel, Merck’s MMRII product insert states that effectiveness and safety have not been established in this population.
Contraindications to receiving ProQuad (MMR-V) vaccine documented in Merck’s product insert include:
- Persons who have experienced a severe allergic reaction or anaphylaxis to any MMR-V vaccine component, including gelatin and neomycin, should not be vaccinated with MMR-V.
- Febrile illness or active untreated tuberculosis
- Persons with acquired or primary immunodeficiency status and individuals receiving immunosuppressive therapy. Vaccination with MMR-V should be delayed for 3 months following the administration of human immune globulin, blood, or plasma.
- Individuals with a family history of hereditary or congenital immunodeficiency.
- Pregnant women.
- Persons with leukemia, lymphoma, blood dyscrasias and other malignant neoplasms affecting the lymphatic systems or bone marrow.
Merck’s ProQuad(MMR-V) product insert warns of a higher incidence of fever and febrile seizures in children between the ages of 12 and 23 months following administration of ProQuad(MMR-V) in comparison with children who receive separate doses of MMR and varicella vaccines. Caution is advised when administering ProQuad(MMR-V) in children with a history of seizures, cerebral injury, or any other medical condition where stress from fever should be avoided.
Both live measles and mumps vaccine are manufactured in chick embryo cell culture. Extreme caution should be taken when vaccinating individuals with a history of anaphylaxis or immediate hypersensitivity to eggs and Merck advises careful evaluation of the risks and benefits when considering vaccination in this population.
Merck’s ProQuad (MMR-V) vaccine product insert reports that transmission of varicella vaccine virus may occur between vaccine recipients and susceptible contacts, including high risk individuals, resulting in both the development or non-development of varicella-like rash. As a result, Merck cautions that vaccine recipients should attempt to avoid close contact with high-risk individuals. This population includes pregnant women who lack a positive history of illness or vaccination and their newborn infants, any infants born prior to 28 weeks gestation, and any immunocompromised individuals.
Merck also advises careful evaluation of the risk and benefits of vaccination with ProQuad (MMR-V) in children with thrombocytopenia or history of the blood disorder as no clinical data on the development or exacerbation of this condition exists. Thrombocytopenia has been reported following vaccination with MMRII, measles vaccine, varicella vaccine and again following an addition dose of both measles and MMRII vaccines.
The safety or efficiency of ProQuad (MMR-V) has not been determined in children who are infected with human immunodeficiency virus (HIV).
Children between 12 months and 12 years of age who receive ProQuad (MMR-V) vaccine should avoid the use of salicylate (aspirin) or salicylate-containing products for 6 weeks following vaccination due to the risk of Reye Syndrome with aspirin and wild-type varicella disease.
ProQuad (MMR-V) is approved for use in children 12 months to 12 years of age. Children under the age of 1 year or older than 12 years of age should not receive ProQuad vaccine.
Contraindications to receiving the PRIORIX vaccine documented in GlaxoSmithKline’s package insert include:
- Persons who have experienced a severe allergic reaction or anaphylaxis to any PRIORIX vaccine component, or to a previous dose of any measles, mumps, and rubella vaccine, should not be vaccinated with PRIORIX.
- Pregnancy
- Immunosuppression
The PRIORIX package insert warns that febrile seizures can occur following administration. Thrombocytopenia and thrombocytopenic purpura have also been reported following vaccination. The tip caps of prefilled syringes contain natural latex and may cause allergic reactions. As with all injectable vaccines, PRIORIX may cause syncope (fainting), which could lead to serious harm. Precautions should be taken to ensure the safety of individuals receiving vaccines.
PRIORIX is approved for use in individuals 12 months of age and older. Children under the age of 12 months should not receive PRIORIX.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
What questions should I ask my doctor about the Mumps vaccine?

NVIC’s If You Vaccinate, Ask 8! Webpage downloadable brochure suggests asking eight questions before you make a vaccination decision for yourself, or for your child. If you review these questions before your appointment, you will be better prepared to ask your doctor questions. Also make sure that the nurse or doctor gives you the relevant Vaccine Information Statement (VIS) for the vaccine or vaccines you are considering well ahead of time to allow you to review it before you or your child gets vaccinated. Copies of VIS for each vaccine are also available on the CDC's website and there is a link to the VIS for MMR and MMR-V vaccines on NVIC's Mumps Quick Facts page.
It is also a good idea to read the vaccine manufacturer product insert that can be obtained from your doctor or public health clinic because federal law requires drug companies marketing vaccines to include certain kinds of vaccine benefit, risk and use information in product information inserts that may not be available in other published information. Vaccine product inserts are located on the Food and Drug Administration’s website and linked in NVIC’s Mumps Quick Facts page.
Other questions that may be useful to discuss with your doctor before getting the mumps (MMR or MMR-V) vaccine are:
- If other vaccines in addition to MMR/MMR-V vaccine are scheduled for my child at this office visit, am I allowed to modify the schedule so fewer vaccines are given at once?
- What should I do if my child has a high fever or appears very ill after vaccination?
- What other kinds of reaction symptoms should I call to report after MMR/MMR-V vaccination?
- If the MMR/MMR-V vaccine doesn’t protect my child, do I have any other options for preventing mumps infection?
Under the National Childhood Vaccine Injury Act of 1986, doctors and all vaccine providers are legally required to give you vaccine benefit and risk information before vaccination; record serious health problems following vaccination in the permanent medical record; keep a permanent record of all vaccines given, including the manufacturer’s name and lot number; and report serious health problems, injuries and deaths that follow vaccination to VAERS.
Remember, if you choose to vaccinate, always keep a written record of exactly which shots/vaccines you or your child have received, including the manufacturer’s name and vaccine lot number. Write down and describe in detail any serious health problems that develop after vaccination, and keep vaccination records in a file you can access easily.
It also is important to be able to recognize a vaccine reaction and seek immediate medical attention if the reaction appears serious, as well as know how to make a vaccine reaction report to federal health officials at the Vaccine Adverse Reporting System (VAERS). NVIC’s Report Vaccine Reactions—It’s the Law webpage can help you file a vaccine reaction report yourself to VAERS if your doctor fails or refuses to make a report.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
NVIC Press Releases, Statements, and Commentaries Related to Mumps
The Vaccine Reaction
- Raines K. Mumps Cases Jump 700 Percent in Northern Ireland. Mar. 11, 2020.
- Fisher BL. Another MMR Failure With Mumps Outbreak in Florida. June 7, 2019.
- TVR Staff. Mumps-like Parotitis Strikes Crew of U.S. Navy Ship. Mar.14, 2019.
- Dillingham S. Minnesota Mumps Outbreak Largest Since 2006. June 10, 2017.
- Cáceres M. Infectious Disease Outbreaks: Are the Vaccines to Blame? May 11, 2017.
- Cáceres M. Scapegoating Anti-Vaxxers for the Mumps Outbreaks: How Predictable. Dec. 23, 2016.
- Mercola J. Mumps Being Spread by and Among Vaccinated People. May 16, 2016.
- Cáceres M. Merck Fraud Case Raises MMR Efficacy Questions. June 9, 2015.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Additional Bibliography of References
Manufacturer Product Information Inserts:
- Merck MMRII - Measles, Mumps and Rubella Virus Vaccine, Live
- Merck ProQuad - Measles, Mumps, Rubella and Varicella Virus Vaccine Live
- GlaxoSmithKline Biologicals SA PRIORIX - Measles, Mumps and Rubella Vaccine, Live
Centers for Disease Control (CDC)
- McKay SL, Kambui A, Taulung LA, et al. Notes From The Field: Mumps Outbreak in a Recently Vaccinated Population — Kosrae, Federated States of Micronesia, August–December, 2017. MMWR 1, 2019; 68(4):95–96.
- Cervantes D, Honza H, Lynch D, et al. Notes from the Field: Mumps Outbreak Associated with Cheerleading Competitions — North Texas, December 2016–February 2017. MMWR 2018;67(36);1019–1020.
- Bonwitt J, Kawakami V, Wharton A, et al. Notes from the Field: Absence of Asymptomatic Mumps Virus Shedding Among Vaccinated College Students During a Mumps Outbreak — Washington, February–June 2017. MMWR 2017;66:1307-1308.
- Donahue M, Schneider A, Ukegbu U, et al. Complications of Mumps During a University Outbreak Among Students Who Had Received 2 Doses of Measles-Mumps-Rubella Vaccine — Iowa, July 2015–May 2016.MMWR 2017;66(14):390-391.
- Albertson JP, Clegg WJ, Reid HD, et al. Mumps Outbreak at a University and Recommendation for a Third Dose of Measles-Mumps-Rubella Vaccine – Illinois, 2015-2016. MMWR 2016;65(29):731-4.
- US Centers for Disease Control and Prevention. Mumps outbreak on a university campus—California, 2011. MMWR 2012;61(48):986-9.
- US Centers for Disease Control and Prevention. Update: mumps outbreak — New York and New Jersey, June 2009–January 2010. MMWR 2009;59(05):125-9.
- US Centers for Disease Control and Prevention. Mumps outbreak — New York, New Jersey, Quebec, 2009. MMWR 2009;58(45):1270-4.
- US Centers for Disease Control and Prevention. Update: multistate outbreak of mumps — United States, January 1-May 2, 2006. MMWR 2006;55(20):559-63.
- US Centers for Disease Control and Prevention. Mumps epidemic — Iowa, 2006. MMWR 2006;55(13):366-8.
- CDC Press Briefing on Mumps Outbreak In the Midwest with Dr. Julie Gerberding, and Dr. Jane Seward - April 2006
- US Centers for Disease Control and Prevention. Mumps outbreak at a summer camp — New York, 2005. MMWR 2006;55(07):175-7.
- US Centers for Disease Control and Prevention. Mumps epidemic — United Kingdom, 2004-2005. MMWR 2006;55(07):173-5.
Selected Media Articles
- Mulder JT. Syracuse University mumps outbreak: Whistleblowers say vaccine is flawed. com Nov. 14, 2017 (updated Jan. 4, 2019)
- Pierson M. Merck accused of stonewalling in mumps vaccine antitrust lawsuit. Reuters Jun 4, 2015.
- Melkie J. Rise in mumps cases linked to waning immunity given by MMR vaccine.The Guardian Jul 4, 2013.
- Koleva G, Merck Whistleblower Suit A Boon to Vaccine Foes Even As It Stresses Importance of Vaccines. Forbes 27, 2012.
Medical Literature
- Fields VS, Safi H, Waters C et al. Mumps in a highly vaccinated Marshallese community in Arkansas, USA: an outbreak report. Lancet Infect Dis. February 2019;19(2):185-192.
- Veneti L, Borgen K, Borge KS et al. Large outbreak of mumps virus genotype G among vaccinated students in Norway, 2015 to 2016. Euro Surveill. September 2018;23(38):
- Principi N, Esposito S. Mumps outbreaks: A problem in need of solutions. J Infect June 2018;76(6):503-506.
- Lewnard JA, Grad, YH Vaccine waning and mumps re-emergence in the United States Sci Transl Med. March 2018; 10(433): eaao5945.
- Fiebelkorn AP, Coleman LA, Belongia EA et al. Mumps antibody response in young adults after a third dose of measles-mumps-rubella vaccine. Open Forum Infect Dis. October 2014;1(3):ofu094.
- Nelson GE, Aguon A, Valencia E, et al. Epidemiology of a mumps outbreak in a highly vaccinated island population and use of a third dose of measles-mumps-rubella vaccine for outbreak control--Guam 2009 to 2010. Pediatr Infect Dis J. April 2013;32(4):374-80.
- Choi KM. Reemergence of mumps. Korean J Pediatr May 2010; 53(5): 623–628.
- Anderson LJ, Seward JF. Mumps epidemiology and immunity: the anatomy of a modern epidemic. Pediatr Infect Dis J. October 2008;27(10 Suppl):S75-9.
- Asatryan A, Pool V, Chen RT, et al. Live attenuated measles and mumps viral strain-containing vaccines and hearing loss: Vaccine Adverse Event Reporting System (VAERS), United States, 1990--2003. Vaccine February 2008; 26(9):1166-72.
- Dayan GH, Quinlisk MP, Parker AA et al. Recent resurgence of mumps in the United States. N Engl J Med. April 2008;358(15):1580-9.
- Dayan GH, Rubin S. Mumps outbreaks in vaccinated populations: are available mumps vaccines effective enough to prevent outbreaks? Infect. Dis. December 2008; 47: 1458–1467.
- Bakshi N, Lawson J, Hanson R, et al. Fatal Mumps Meningoencephalitis in a Child With Severe Combined Immunodeficiency After Bone Marrow Transplantation J Child Neurol 11(2): 159-162.
- Geier, MR., Geier DA. Pediatric MMR vaccination safety. Int Pediatr. February 2003;18(2):203-208.
- Miller E, Goldacre M, Pugh S et al. Risk of aseptic meningitis after measles, mumps, and rubella vaccine in UK children. Lancet April 1993;341(8851):979-82.
- Nabe-Nielsen J, Walter B. Unilateral total deafness as a complication of the measles-mumps-rubella vaccination. Scand Audiol Suppl 1988;30:69-70.
- Stewart BJ, Prabhu PU, Reports of sensorineural deafness after measles, mumps, and rubella immunisation. Arch Dis Child July 1993; 69(1): 153–154.
- Otten A, Helmke K, Stief T, et al. Mumps, mumps vaccination, islet cell antibodies and the first manifestation of diabetes mellitus type I. Behring Inst Mitt July 1984;(75):83-8.
- Poling JS, Frye RE, Shoffner J, et al. Developmental Regression and Mitochondrial Dysfunction in a Child With Autism J Child Neurol February 2006; 21(2): 170–172.
- Sinaniotis CA, Daskalopoulou E, Lapatsanis P, et al. Letter: Diabetes mellitus after mumps vaccination. Arch Dis Child September 1975; 50(9): 749–750.
- MacDonald SE, Dover DC, Simmonds KA, et al. Risk of febrile seizures after first dose of measles–mumps–rubella–varicella vaccine: a population-based cohort study. CMAJ August 2014; 186(11): 824–829.
- Ma SJ, Xiong YQ, Jiang LN et al. Risk of febrile seizure after measles-mumps-rubella-varicella vaccine: A systematic review and meta-analysis. Vaccine July 2015;33(31):3636-49.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Mumps & Mumps Vaccine Quick Facts
Mumps

- Mumps is a contagious viral infection. Symptoms begin with a headache, muscle aches, tiredness, and loss of appetite. A stiff is a sign that aseptic meningitis (inflammation of the lining of the brain) may have developed, which is a rare complication of mumps ;
- Mumps virus can be found in the saliva, throat and urine of an infected person. The virus is usually spread through the air by respiratory droplets or by contact with the saliva of an infected person :
- The time between when a person comes into contact with a person infected with mumps and first begins to experience symptoms of mumps (incubation period) ranges from 12 to 25 days . The illness lasts for an average 7-10 days, but may last longer before symptoms completely disappear ;
- The typical “signature” physical sign of mumps is visible swelling of one or both sides of the face under the ears and chin. Males, who are past puberty, can experience pain and extreme swelling of the testes and rarely, become sterile. Mumps is very rarely fatal;
- There is no specific treatment for mumps except alleviation of symptoms with rest, pain relievers and cool compresses.
- Three mumps containing vaccines are available in the U.S. Two vaccines, MMRII and PRIORIX, contain live attenuated measles, mumps, and rubella virus. The third, ProQuad (MMR-V), contains live attenuated measles, mumps, rubella, and varicella virus. PRIORIX, ProQuad, and MMRII were developed using aborted fetal cell lines. The CDC recommends that children get two doses of a Mumps containing vaccine with the first dose given between ages 12-15 months, and the second dose given between ages 4-6 years. All mumps vaccines are contraindicated during pregnancy.
- Common side effects from the MMR or MMR-V vaccine include low-grade fever, skin rash, itching, hives, swelling, reddening of skin, and weakness. Reported serious adverse reactions following MMR and MMR-V vaccination include seizures, brain inflammation and encephalopathy; thrombocytopenia; joint, muscle and nerve pain; gastrointestinal disorders; measles like rash; conjunctivitis and other serious health problems. A vaccine strain infection following vaccination may be the cause of the full body rash.
- Since 2006, multiple outbreaks of mumps have occurred in the U.S. and abroad in vaccinated children and young adults, occurring often on college campuses. In 2017, the CDC recommended a third dose of a mumps containing vaccine to be administered in the event of an outbreak. Numerous studies examining mumps outbreaks that have occurred in highly vaccinated populations have experts suggesting that both the waning of vaccine induced immunity and an ineffective mumps vaccine may be to blame.
- In 2010, new information questioning the efficacy of the mumps portion of MMR vaccine emerged when two former Merck employees filed a lawsuit alleging the company altered testing results and studies to make the mumps vaccine in MMR appear to be more effective than it really is in preventing mumps infection. Court proceedings on the case are still pending.
- As of April 1, 2024, there had been 1,198 claims filed in the federal Vaccine Injury Compensation Program (VICP) for injuries and deaths following mumps-containing vaccination, including 66 deaths and 1,132 serious injuries.
- Using the MedAlerts search engine, as of March 29, 2024, there have been 111,951 reports of mumps vaccine reactions, hospitalizations, injuries and deaths following mumps vaccination made to the federal Vaccine Adverse Events Reporting System (VAERS), including 524 related deaths, 8,489 hospitalizations, and 2,140 related disabilities.
- Measles, Mumps and Rubella Vaccine, Live (MMRII). Product Insert & Licensing Information
- Measles, Mumps and Rubella Vaccine, Live (PRIORIX). Product Insert & Licensing Information
- Measles, Mumps, Rubella and Varicella Virus Vaccine Live (ProQuad). Product Insert & Licensing Information
- CDC on Mumps
- CDC on Mumps Vaccination
- Mumps Vaccine Information Statement (VIS) MMR
- Mumps Vaccine Information Statement (VIS) MMR-V (ProQuad)
Vaccine Reaction Symptoms & Ingredients
Our Ask 8, If You Vaccinate webpage contains vaccine reaction symptoms and more.
Search for Vaccine Reactions
NVIC hosts MedAlerts, a powerful VAERS database search engine. MedAlerts examines symptoms, reactions, vaccines, dates, places, and more.
Reporting a Vaccine Reaction
Since 1982 NVIC has operated a Vaccine Reaction Registry, which has served as a watchdog on VAERS. Reporting vaccine reactions to VAERS is the law. If your doctor will not report a reaction, you have the right to report a suspected vaccine reaction to VAERS.
IMPORTANT NOTE: NVIC encourages you to become fully informed about Mumps and the Mumps vaccine by reading all sections in the Table of Contents, which contain many links and resources such as the manufacturer product information inserts, and to speak with one or more trusted health care professionals before making a vaccination decision for yourself or your child. This information is for educational purposes only and is not intended as medical advice.
Mumps Outbreak 2006
According to the CDC, the multi-state outbreak of mumps in 2006 resulted in 6584 cases of mumps. Most of those affected were Midwestern college students living in dormitories.
As Dr. Gerberding points out in her press conference (see transcript below): "Fortunately, [mumps] is usually not a serious disease" and complications are "rare."
Press Briefing on Mumps Outbreak In the Midwest with Dr. Julie Gerberding, and Dr. Jane Seward
Source: http://www.cdc.gov/media/transcripts/t060419.htm
DATE: WEDNESDAY, APRIL 19, 2006
DR. GERBERDING: Good afternoon. Thank you for taking time to join us for this press conference on mumps. We know that there is a large outbreak of mumps going on mainly in the Midwestern part of our country, and this has been a tough time for college students and their parents and the people who are responsible for containing this problem. This actually is the largest outbreak of mumps that we have seen in this country in more than 20 years. We have more than a thousand cases reported from eight states, and we also have additional cases undergoing investigation in seven more states. We are not going to be surprised if there are more cases in more states just given the nature of mumps and the way this outbreak is progressing.
I really want to take a moment to thank the public health officials in the affected states, including Iowa that has been the hardest hit. These individuals have been working tirelessly to try to ascertain the source of the outbreak, work hard to take the steps to contain it, and also working very, very hard to provide information and updates on an ongoing basis, all in a very fast track. So we really applaud and appreciate, as always, our colleagues in the state and local public health systems.
We would like to just say a couple of things about mumps just for people who are not familiar with it. Fortunately, most people are not familiar with mumps because we have had a vaccine since 1967 and that vaccine has largely eliminated frequent outbreaks of mumps in our country. Mumps is a virus disease. It generally affects the body with fever, headache, and tiredness, the kinds of virus-like illness that we get with most of the common viruses. But it has a very special tendency to cause inflammation in your saliva glands, so people get the big, puffed cheeks from the involvement of those glands.
Fortunately, it is usually not a serious disease. People are usually ill for a week or so. But, in some people, it can have serious complications. Up to 10 percent of people will develop meningitis, a certain proportion of people will develop orchitis, that's an inflammation of the testes, which in adolescent boys or older men can sometimes lead to infertility. It can also involve other tissues. It has been reported to be associated with spontaneous abortion and potentially deafness. So although these complications are very rare, occasionally they are serious and our understanding is right now there have been at least 20 hospitalizations associated with this outbreak, but, fortunately, so far no deaths.
I have to emphasize that the best protection against mumps is the vaccine. There is a lot of confusion right now about whether or not this outbreak is related to some problem with the vaccine, and I really want to emphasize that while we are of course investigating the outbreak and we will learn more about the efficacy of the vaccine in this particular setting, we have absolutely no information to suggest that there is any problem with the vaccine.
The problem here is with the lack of complete coverage of the vaccine, number one. Our vaccine program for mumps began in 1967, but just by nature, there is a group of students, roughly college-age students, who may be less likely to have received both doses of the mumps vaccine and are incompletely vaccinated. Therefore, they are susceptible when infection is introduced, and they have a very high chance of getting mumps under those environments.
In addition, although this is a very good vaccine, it is not perfect. About 10 percent of people who get both doses of the vaccine still remain [susceptible] to mumps. So if you are in a community of 10,000 people and 10 percent of the people who got both doses of the vaccine are susceptible, once you get a little outbreak going in that community, that means that up to 1,000 people in the community would actually come down with mumps even though they were properly immunized with what we know is a very good vaccine.
What is going on here in the context of the outbreaks is a number of people who have not received both doses, coupled together with people who have received the vaccine but are susceptible anyway because it is not perfect, living in crowded conditions like college dormitories, or mixing up with other students such as might happen during spring break or the holidays, and really setting off a cascade of transmissions that is going to take a while to curtail and eventually stop. The most important step that is being taken in the affected areas is to implement vaccine programs for those people who have not received both doses. For individuals who are in the school age population, for individuals who are post-high school in the school environment such universities or colleges, or people in institutions, and very importantly, for health-care workers, if you have not received two doses of the mumps vaccine, it is very important that you get your second dose. The state health officials in the affected areas are working hard to develop plans and to support the access to the school in this context.
CDC is taking some steps to support the states. We do have disease detectives who are working on assisting and helping to study some of the features of the outbreak so we have a better idea and can learn from this as the virus spreads from community to community. We are also assisting in the supply of vaccine itself. We are very pleased that we have some mumps vaccine in the form of mumps, measles, and rubella vaccine in our stockpile. Already we have committed to make 25,000 doses of that vaccine available to the state of Iowa to support them as they need it. But in addition, I am very pleased to say that Merck, the company that makes MMR II has donated to CDC, 25,000 doses of vaccine that we will use as we see fit to help support to immunize people in the affected areas. It is a very generous gift, and we are happy to have it because this outbreak is happening somewhat late in our fiscal year and we appreciate the opportunity to have an additional supply to share with those who need it. The vaccine really is the best protection. We again emphasize the importance of getting both doses of the vaccine.
What we expect to happen in the next several weeks is that immunization will increase. The medical community has received information and resources from CDC and from state health officials. A lot of clinicians have never seen a case of mumps, and so we have to remind and refresh them about what mumps looks like. Sometimes mumps is not very symptomatic, and not everyone gets the swollen glands, so it can be confused with other viral illness. Fortunately, our laboratories at the state health department level have the special test necessary to rapidly diagnose mumps, so they are playing a very key role in this investigation. We are encouraging clinicians who have any suspicion about cases to check with their local health officials to make sure that they are doing everything they need to do to ascertain whether a case exists or not. But we will not be surprised if we see more people affected either in the college context or as students who spend time with their families or with their community friends, we will continue to see some extension of this outbreak into the community level and we need to be prepared for that, and if things change or if the pattern changes beyond what we expect, certainly you can look forward to further updates from CDC as well as further updates from health officials at the local level who are really on the front line of this and who are in the best position to be able to advise the community about what specifically should be done in their community.
Let me stop now and take some questions. I'll take one from the room first.
QUESTION: [Off mike.]
DR. GERBERDING: There are several different methods for determining vaccine efficacy, but when we have cases of mumps, the first question to ask is, is this person not vaccinated, have they had one dose of vaccine or do they have two doses of vaccine? One thing we can do is compare people with mumps to people like them who did not have mumps, and by comparing the frequency of vaccination in the people who have the disease with people who do not have the disease, we have tools that allow us to estimate vaccine efficacy in that context. So that really is the simplest way, and those are the kinds of things that our teams are looking at with the state health officials in the field as we speak.
QUESTION: [Off mike.]
DR. GERBERDING: The question is do we have initial results, no, and you can expect that as this is an unfolding story, that will take some time. One of the complications is that most people do not have electronic health records, and so ascertaining vaccine status particularly from childhood vaccines, is something that we do not have rapid tools to accomplish right now. Until we have electronic health records, we are going to have to do this the old-fashioned way, by mom's recall sometimes, and by searching through hard copies of medical records which are difficult to get one's hands on. So expect this to take a bit of time, but right now we are not seeing anything surprising in this pattern of transmission that would suggest that there is a vaccine problem, per se, it is a problem of complete coverage of the vaccine and the fact that even when the vaccine is optimal, it is never 100-percent protective.
I will take a telephone question, please.
OPERATOR: Thank you, Doctor. Nicole Aksamit with Omaha World Herald, your line is now open.
MS. AKSAMIT: Dr. Gerberding, in Iowa we know the largest age group affected is roughly between the ages of 18 and 22. From the data that Iowa has presented, it indicates that that is whether or not those people attend college and whether or not they were indeed vaccinated once or twice. We have not seen that particular age population being a spike in the cases so far in Nebraska, and I guess I have not seen age breakdowns in the other states that have cases.
Could you address whether that has been uniform across the board, that there is a spike in college-age people? And if not, what is the running theory on why that age group in Iowa?
DR. GERBERDING: The main reason that college-age people were particularly involved I think early in Iowa is that they are very crowded together and somewhere along the line there was a college campus where someone came down with mumps and it began to spread in the social context of that campus. But you have to think of this as a network, and so when someone leaves the original source of an outbreak and goes to a new community where there are susceptible people, whoever those people are or however old they are, they will be the next to come in contact with the mumps virus. So this is the kind of work that the state health officials are engaged in right now, tracking down the who, what, when, where, why, and how of the outbreak, and we can expect there will be lots of variations on a theme as this work gets conducted.
DR. GERBERDING: I will take another phone question.
OPERATOR: Thank you. Anita Manning from USA Today, your line is now open.
MS. MANNING: Thank you very much. Dr.Gerberding, I am sorry, but I could not hear the first question that you were asked from the room, and I think it had to do with a waning immunity question. If you do not mind, we have seen waning immunity with other vaccines like the pertussis vaccine, and I was just wondering if there is any kind of blood testing or serologic testing going on in the communities there to see if there is a waning immunity.
Then a second question that I have is, is there any evidence that this is spreading by people who are asymptomatic? Is there asymptomatic spreading? Thank you.
DR. GERBERDING: In terms of waning immunity, we have no evidence from any of the information we have so far or from historical experience that waning immunity plays any part. People who receive two doses of mumps vaccine just sometimes simply do not respond to it. Their immunologic system just does not regard that as a source of stimulation to their immune system. If waning immunity were a primary problem, we would expect much older people to be affected, at least those who did not have mumps when they were children. So we are looking into this as one of several possibilities, but I think right now what we know about this vaccine's efficacy, what we know about the undervaccinated people in this age cohort, and what we know about the sociology of life in some of these community settings, we have ample explanation for why the virus is spreading the way it is.
It is possible for asymptomatic people to transmit mumps virus. People are sometimes asymptomatic for a significant period of time, and about 20 percent of the time, the cases are mild or asymptomatic, so the disease can be transmitted in the context of a person who does not recognize that they have mumps, all the more reason, again, for people who have not received both doses to get their vaccines.
Also, people who are going to have symptomatic mumps are sometimes able to transmit the virus for 2 or 3 days before they actually have it. So there are several reasons why it is sometimes difficult to completely immediately contain the problem because of the asymptomatic nature of the spread.
One of the other important aspects of containment in addition to the vaccine is that people who do have mumps should remain isolated during their period of contiguousness. This is very important, so that they are not out and about, and particularly college campuses, that they are not exposing other students, or if they are health-care workers, they are not exposing other health-care workers or patients. So it is important to follow the local health official's advice about isolate the people who are suspected or known to have mumps.
DR. GERBERDING: I can take another telephone question, please.
OPERATOR: Thank you. Rob Stein from the Washington Post, your line is now open.
MR. STEIN: Thanks very much for doing this. I have a couple of questions. The first one was that you said 1,000 cases in eight states. I was wondering if you could give us the breakdown on how many of those are in Iowa and how many of those are in the other states, and what those other states are.
Then the thing is, is the outbreak continuing at the same rate? Is it slowing down? Is it accelerating? Do you have any sense of where things stand in getting it contained?
DR. GERBERDING: I do not think this outbreak is at steady state, so we can expect some variability and some stuttering as it moves forward. There are 815 reported cases in Iowa, at least as of our last communication with the Iowa State Health Department, so that by far is the largest number of cases that we are aware of so far.
There are 350 cases reported from seven other states, which includes Minnesota, Kansas, Illinois, Nebraska, Wisconsin, Missouri, and Oklahoma, and if you did not get that, we can circle back to you with that information. Then there are a handful of other states that may have cases, but they just have not confirmed them as probable or definite cases and so they are still a work in progress.
The epidemiology or the characteristics of the cases in all of these areas is still undergoing an active investigation. As I said, many clinicians have not been familiar with mumps because they have not really seen cases in recent years, so part of this is as clinicians become more familiar or more aware of what we are looking for, we would expect to see more cases just because there are more cases being diagnosed. So a work in progress, and we will try to provide updated information on this as states make their reported cases available to CDC. Other questions here?
QUESTION: [Off mike] outbreak, what should people be doing as far as protecting themselves? Should they be seeking the vaccine, and is there enough vaccine if people go out and try to get those second doses in [off mike]
DR. GERBERDING: CDC has sent a health alert to the health officials and clinicians reminding people that generically it is important to encourage second-dose vaccinations for those people who do not have it. Fortunately, because we have such high coverage rates among younger children for vaccines, most children in our country actually have received both doses of the mumps vaccine. Older people have had mumps, we just have this transition zone going on where there will be significant numbers of people, and we do not know exactly what proportion of people in this age group have not received both doses. That is another one of the estimates. It is probably not constant from area or population to population.
We have significant numbers of doses in two of the CDC stockpiles, in a stockpile called Vaccines for Children. That vaccine can be used for kids up to 18 years of age. We also have stockpiled vaccine in our 317 Vaccine Stockpile which can be used for emergencies for whomever needs it, and we will not hesitate to use the vaccine that we have stockpiled as we need it, and if we need more, we can ask for flexibility in the use of what we have, and also the manufacturers of it have indicated cooperation, as Merck demonstrated by its donation today. So right now we are not anticipating in the short-run a vaccine shortage problem, but, of course, as this expands and goes on, we will keep you updated as the supply and demand issues unfold.
DR. GERBERDING: Another question from the phone?
OPERATOR: Thank you. Karen Shideler with the Wichita Eagle, your line is now open.
MS. SHIDELER: Thanks for your help today. I have two quick questions. Number one, you mentioned older people who have had the mumps. I know that is not the case because in our news room there have been several who have come up and asked, Should I go get the shot now. My second part of that question is, is there a way to tell whether or not you are in the 10 percent of who the vaccine did not take?
DR. GERBERDING: Right now there is not an easy test for determining whether or not if you have had the vaccine, you are susceptible. There is an antibody test for mumps, but the relationship between that test result and susceptibility is not completely ascertained. There is a lot of science that needs to be done in looking at that particular measure, and it is not something that is clinically useful or available to people at this point in time.
For people who have had mumps, it is important to remember that we assume that you are now completely protected against mumps, so having had mumps as a child is in this context a safeguard against an additional case. It would be unusual for someone to get mumps twice, it has probably happened, but it would not be a concern in the context of the outbreak that we are experiencing.
It is, again, just so important to emphasize that one of the lessons learned about this particular outbreak is that vaccine coverage matters especially when we are dealing with a vaccine that isn’t 100-percent efficacious, that even a slight reduction in the coverage that we could achieve can create an environment where it is even more likely for the mumps virus to spread. So fortunately we are not seeing outbreaks right now in schools or in younger children in large part because they have a higher degree of two-dose coverage. That does not mean it could not happen, because there will be children in school, of course, who have not completely responded to the vaccine. But it just a reminder of what happens when we have any lag in our immunization coverage for people.
QUESTION: Is this outbreak connected to the one in the United Kingdom?
DR. GERBERDING: We do not know yet about the relationship of how this virus got introduced. In past outbreaks of mumps, there was a very large outbreak of mumps in the United Kingdom. They are particularly related, again, both to the vaccine efficacy, but also the much lower coverage rates in the population, so they had more than 100,000 cases of mumps in a country that is much smaller than the United States, and that is a very sobering reminder of why vaccine coverage matters.
We do know that the genotype at least in the early cases of this outbreak was the same genotype of virus that was associated with the United Kingdom outbreak, but that does not necessarily mean there was a direct link to introduction. It is certainly possible, but we do not have any proof of that at this point in time.
DR. GERBERDING: I will take another phone question, please.
OPERATOR:Thank you, Jeremy Manier from the Chicago Tribune, your line is now open.
MR. MANIER: Thanks very much. I do not mean to downplay what you said about the importance of vaccine coverage, but in this case, I wonder if it is really applicable to this outbreak. The figures that Iowa has released so far anyway are that only 3 percent of the people they have studied had no dose of the vaccine at all, and 65 percent they think had two doses. I am not saying that that does not play a role, but in this I am not sure how it comes into play.
Also, the second part of this, are you concerned that the problem here could get to the same kind of scale as the U.K. has seen? What would it take for this to become something that evolved into tens of thousands of cases over a period of years as they have?
DR. GERBERDING: As I said, this is an unstable situation right now and we are not able to reliably predict where this will go. We do know what is important about containment, and we are doing everything we can to support the state health officials who are responsible for executing those steps. I do not want to second-guess the specific elements of the containment and preparation activities that are ongoing in the state because every jurisdiction does have a little bit different epidemiology and a little bit different problem to solve. We do expect more cases, absolutely. We hope that the steps taken to isolate infected people as well as vaccinate to raise the general level of protection will definitely help slow this down. We have seen that successfully work in the past, and we hope that it would be successful this time.
In any population, your question about the importance of undervaccinated people, it is a function of how many undervaccinated people there are, if the people who have only received are only 65 or 80 percent protected, then obviously if the mumps virus is introduced and there are a lot of those people, it is going to take off much faster and there will be a very vulnerable group of people versus a smaller percentage of people who are vulnerable because the vaccine was not working, but a small percentage of large number of people is still a lot of susceptibles. So if you have 100,000 people and 10 percent of them did not respond to the vaccine, that is still a very large proportion of people, and if that population is large compared to those who have only had one does, then you will see that the statistics represent the majority of cases being in the two-dose vaccinated population.
So we are seeing pretty much what our math would suggest, but some of them work that we are doing in these areas is to really dig into these numbers and try to understand the transmission dynamics with more precision, and I think we can give a better you a better answer to your question when those data become available.
I would ask if our mumps expert, Dr.Seward, would have anything to add to that. If you would just come up to the microphone, Jane. This individual is the world's treasure house of information on mumps, so she has been very busy lately.
DR. SEWARD: I am Dr. Jane Seward, Division of Viral Diseases, CDC. The one information I would add is that the attack rates that we are seeing when we look at a very specific case definition for mumps was parotitis in these two-dose vaccinated colleges is quite low, and much, much lower than attack rates in high schools 20 years ago. So that would lead us to believe, again, that we are getting very good protection from the MMR vaccine. As Dr. Gerberding explained, the reason such a high proportion of cases have received two doses is because such a high proportion in the population have received two doses of MMR vaccine.
DR. GERBERDING: I will take another question from the phone, please.
OPERATOR: Thank you. Tony Leys from The Des Moines Register, your line is now open.
MR. LEYS: Doctor, I would like you to compare the danger of this outbreak to previous outbreaks such as West Nile. Also, are you concerned that with the semester is going to be over in the next couple of weeks at a lot of these schools and kids will be going home, are you concerned that that is going to spread it across the country?
DR. GERBERDING: No, we have characterized this as a college-age population of students at risk. It is important to remember this is not just about college students. That just happens to be the largest and earliest hub of transmission that was recognized.
We have seen many opportunities for the first transmission environment to seed the virus into other communities, into families, and into other age groups. So as people went on their Spring Break, as they went for Passover or for Easter, students are highly connected as are most people these days, and so we really cannot predict at this point in time where the virus will go next and what other populations of people we need to be concerned about.
Let me emphasize again, however, that health-care workers are always going to be a special risk population for any communicable disease. It is very important that the health-care professionals who may be taking care of people of any age with mumps be sure to have both doses of the vaccine just to give them that extra oomph of safety.
The answer to your first question, I'm sorry, I forgot your first question, so you will have to repeat it for me.
MR. LEYS: The danger compared to West Nile.
DR. GERBERDING: The comparative risk of this mumps outbreak to other infectious diseases such as West Nile, no, it is really an apples and oranges kind of comparison. We are very early in understanding the scope and magnitude of this problem. We hope that we will soon get to the down slope of the curve, but we do not know that yet, and we just have to ultimately do everything possible to take the steps necessary for containment, and I think in retrospect we will be able to get a full picture of the magnitude of the problem.
One advantage that we are seeing at least so far in this outbreak is that most of the people are young and relatively healthy. We have not had any deaths so far related to this outbreak, so that is less morbidity and mortality than something like West Nile which tends to preferentially cause the most severe disease in the elderly, facilitates a much longer sequelae and sometimes much higher fatality rates. So it is an apples and oranges comparison, and I do not think that we can really say that one is less bad or more bad, but we certainly in all cases will do everything we can to try to contain them.
I am going to just take one more question from the phone, please.
OPERATOR: Thank you. Brian Hartman with ABC News, your line is now open.
MR. HARTMAN: Thank you. Is there any doubt that you will eventually be able to contain this outbreak in fairly short order? And I also wonder if you could give us any detail on the seven states that are under investigation, but you have not confirmed anything.
DR. GERBERDING: Your second question about states, I am not going to discuss states that have not officially reported cases because it is really the province of the states to make the first steps toward identifying and reporting cases, so we are not going to reveal any information before they have been confident that they have accurate perspectives to share.
The whole issue of inability to contain it I think is to some extent a semantic issue. Mumps is not an unheard of disease even in the absence of an outbreak. We have mumps every year. We see cases from time to time. What is unique here is that we have a cycle of transmission that has resulted in a conspicuous outbreak and one that seems to be extending further and further across communities at risk. So we certainly will not see a complete end of mumps when this cycle is broken, but we do hope to reduce the ongoing, rapid transmission in the population that would best be characterized as an outbreak.
Thank you for your interest, and we do commit to giving you updates as more information becomes available. Thank you.
OPERATOR: Thank you. This does conclude today'sconference call. We thank you for your participation. [End of press conference.]



